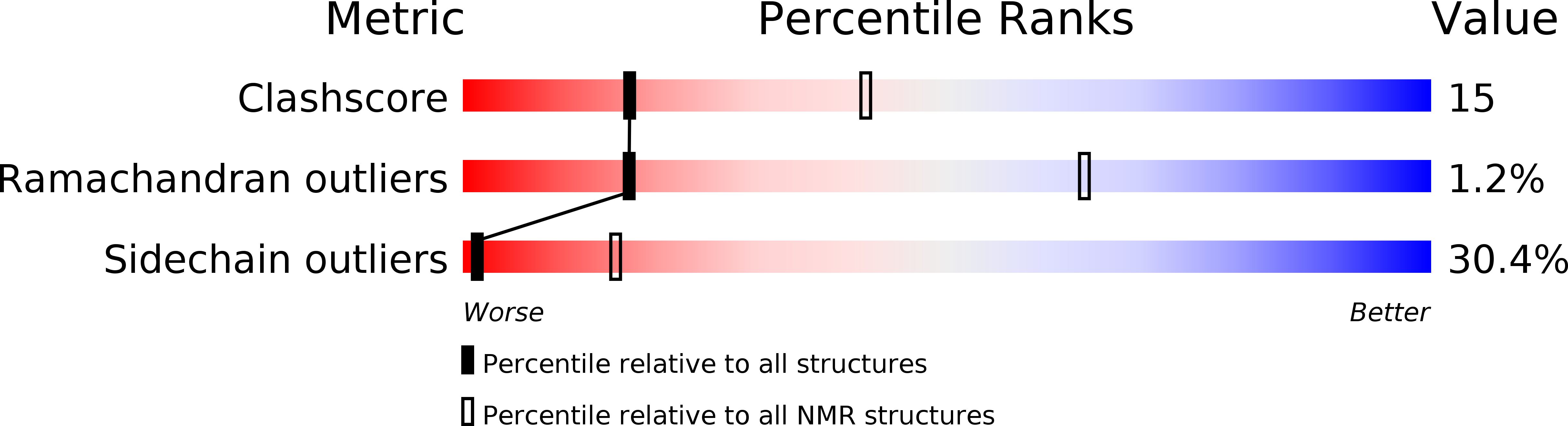
Deposition Date
2013-10-24
Release Date
2013-12-18
Last Version Date
2025-03-26
Entry Detail
PDB ID:
2MFX
Keywords:
Title:
Non-reducible analogues of alpha-conotoxin Vc1.1: [2,8]-cis dicarba Vc1.1
Biological Source:
Source Organism(s):
Conus victoriae (Taxon ID: 319920)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy