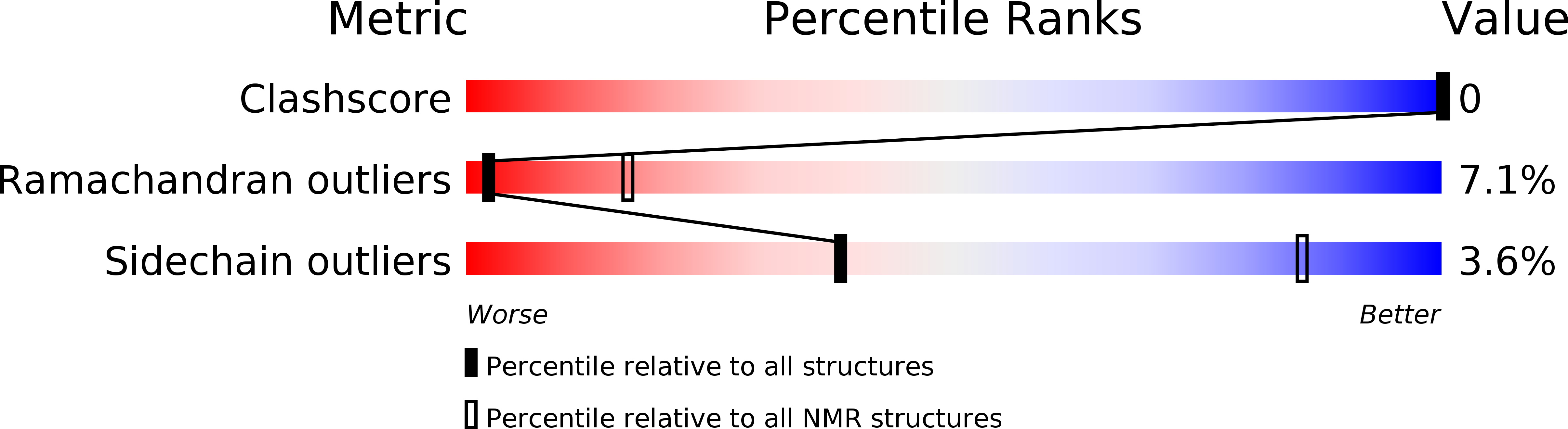
Deposition Date
2013-08-15
Release Date
2013-10-30
Last Version Date
2024-05-01
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
1
Selection Criteria:
target function