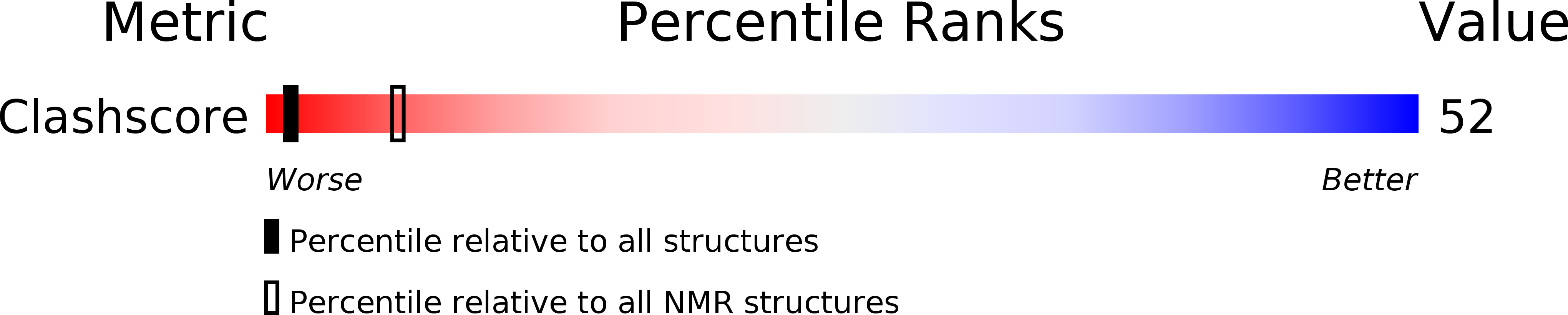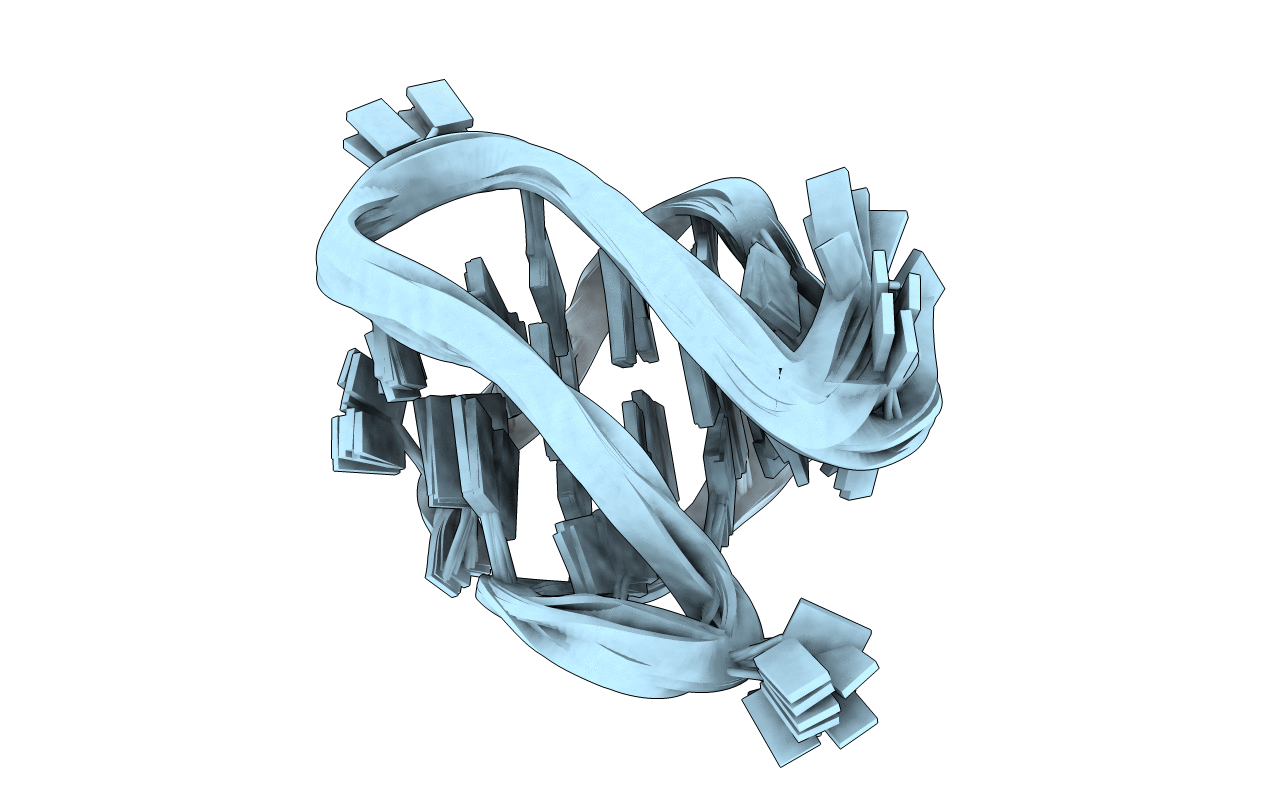
Deposition Date
2013-08-02
Release Date
2013-09-18
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2MBJ
Keywords:
Title:
Structure of an antiparallel (2+2) G-quadruplex formed by human telomeric repeats in Na+ solution (with G22-to-BrG substitution)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy