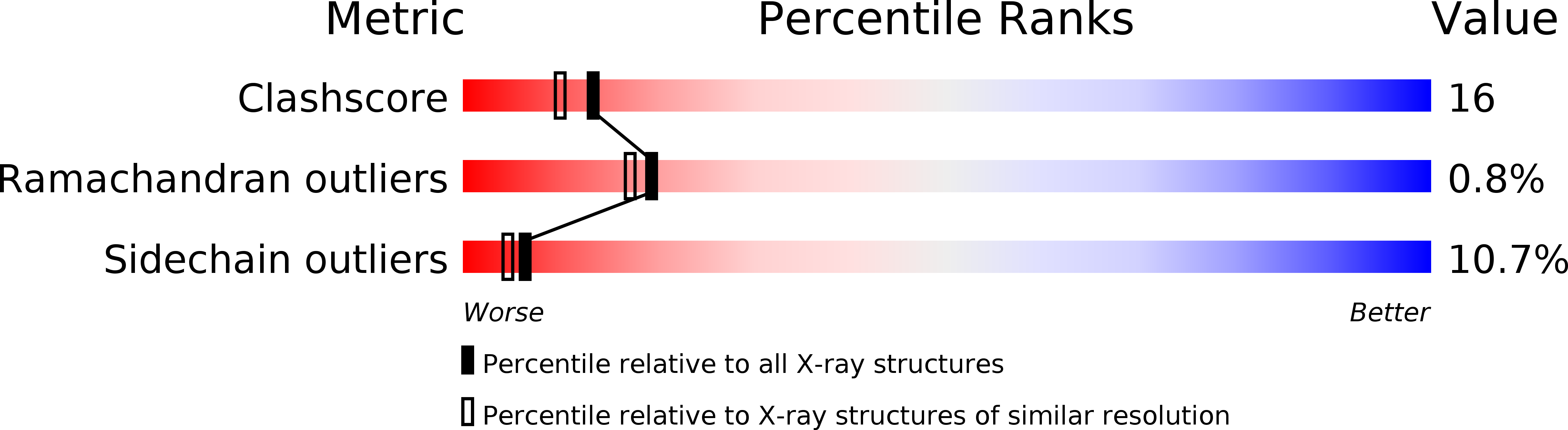Deposition Date
1992-05-20
Release Date
1994-01-31
Last Version Date
2024-06-05
Entry Detail
PDB ID:
2MAD
Keywords:
Title:
THE ACTIVE SITE STRUCTURE OF METHYLAMINE DEHYDROGENASE: HYDRAZINES IDENTIFY C6 AS THE REACTIVE SITE OF THE TRYPTOPHAN DERIVED QUINONE COFACTOR
Biological Source:
Source Organism(s):
Paracoccus versutus (Taxon ID: 34007)
Method Details:
Experimental Method:
Resolution:
2.25 Å
R-Value Observed:
0.20
Space Group:
P 31 2 1