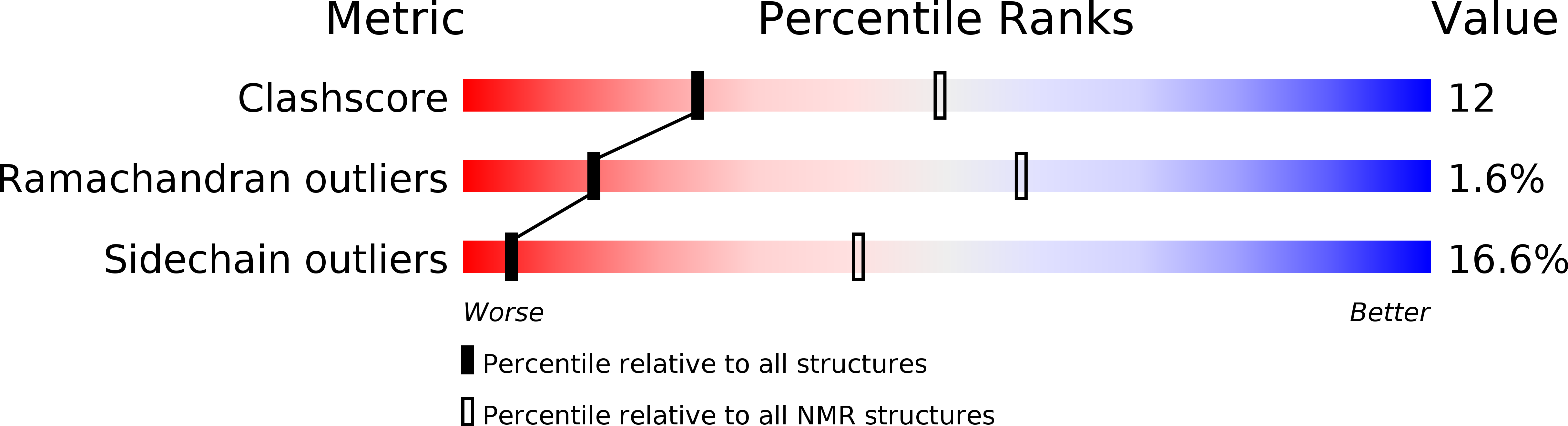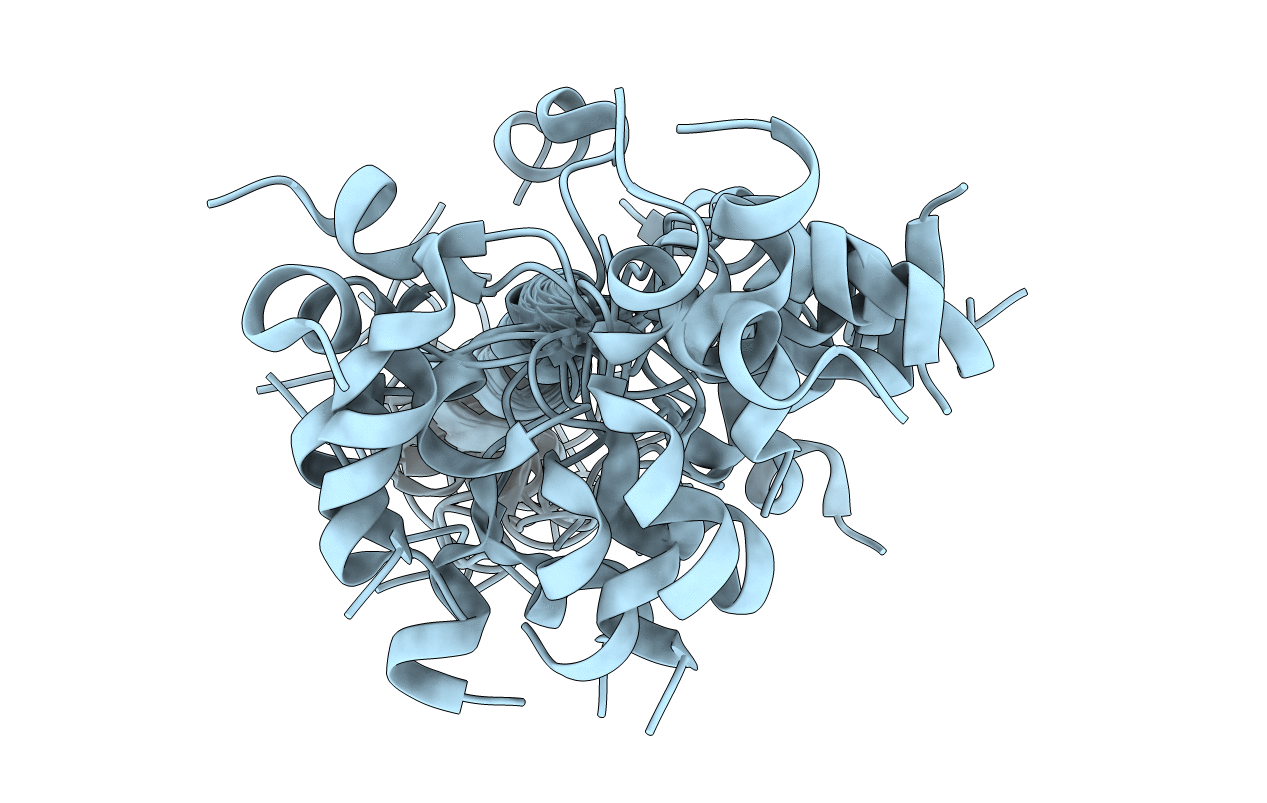
Deposition Date
2013-05-02
Release Date
2013-06-05
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2M7X
Keywords:
Title:
Structural and Functional Analysis of Transmembrane Segment IV of the Salt Tolerance Protein Sod2
Biological Source:
Source Organism(s):
Schizosaccharomyces pombe (Taxon ID: 284812)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
25
Selection Criteria:
structures with the lowest energy