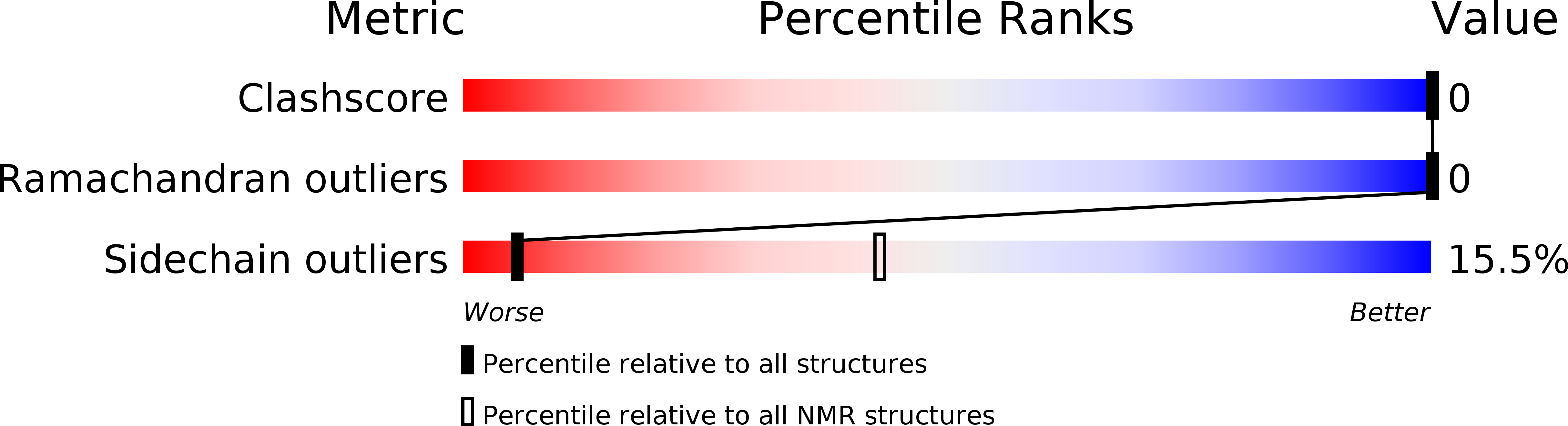Deposition Date
2013-03-28
Release Date
2014-04-02
Last Version Date
2024-10-30
Entry Detail
PDB ID:
2M6A
Keywords:
Title:
NMR spatial structure of the antimicrobial peptide Tk-Amp-X2
Biological Source:
Source Organism(s):
Hordeum vulgare subsp. vulgare (Taxon ID: 112509)
Expression System(s):
Method Details:
Experimental Method:
Conformers Submitted:
10
Selection Criteria:
structures with the least restraint violations