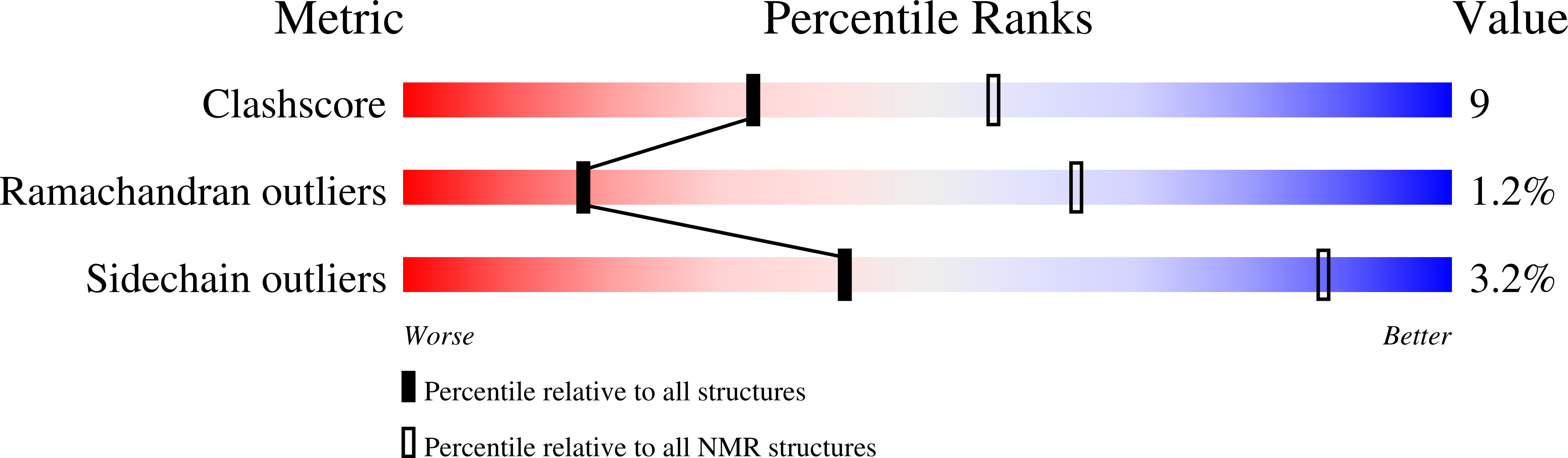
Deposition Date
2013-02-14
Release Date
2013-08-21
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2M56
Keywords:
Title:
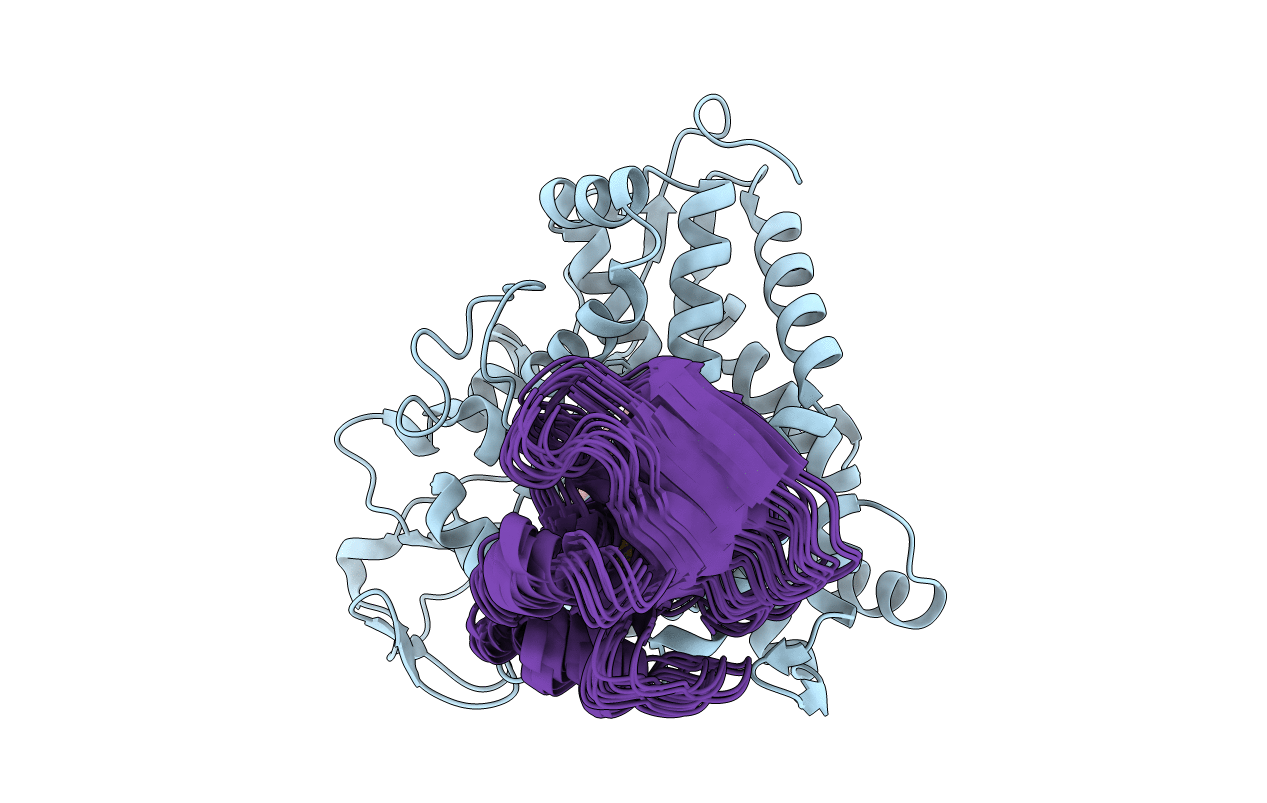
The structure of the complex of cytochrome P450cam and its electron donor putidaredoxin determined by paramagnetic NMR spectroscopy
Biological Source:
Source Organism(s):
Pseudomonas putida (Taxon ID: 303)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the least restraint violations