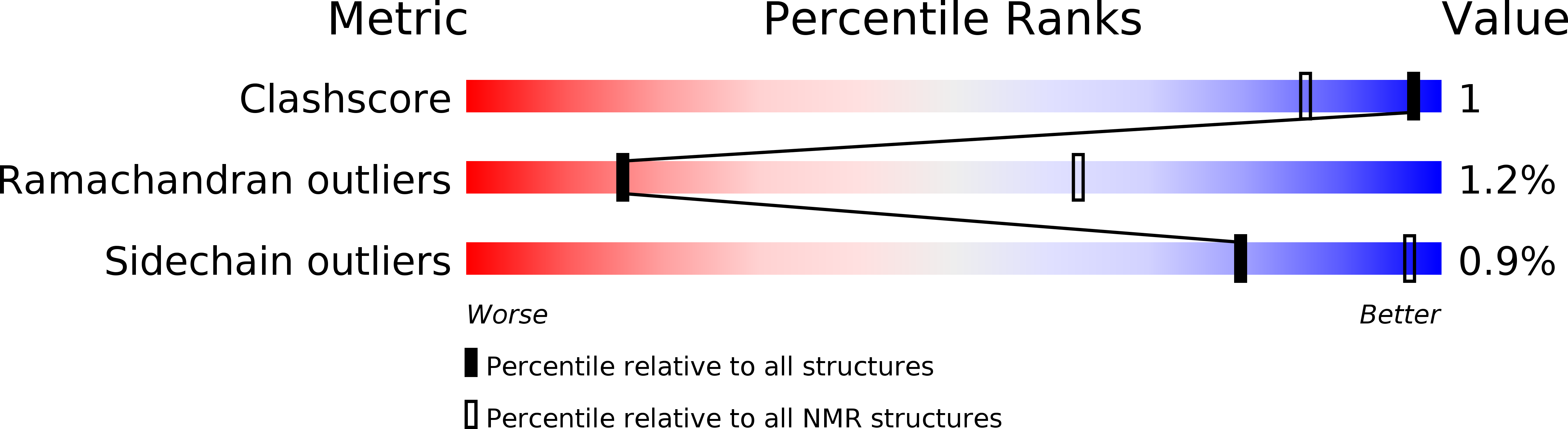Deposition Date
2013-01-20
Release Date
2013-12-04
Last Version Date
2024-10-16
Entry Detail
PDB ID:
2M3I
Keywords:
Title:
Characterization of a Novel Alpha4/6-Conotoxin TxIC from Conus textile that Potently Blocks alpha3beta4 Nicotinic Acetylcholine Receptors
Biological Source:
Source Organism(s):
Conus lividus (Taxon ID: 89426)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
Structures with the lowest MolProbity score