Deposition Date
2012-10-30
Release Date
2012-11-21
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2M0N
Keywords:
Title:
Solution structure of a DUF3349 annotated protein from Mycobacterium abscessus, MAB_3403c. Seattle Structural Genomics Center for Infectious Disease target MyabA.17112.a.A2
Biological Source:
Source Organism(s):
Mycobacterium abscessus (Taxon ID: 561007)
Expression System(s):
Method Details:
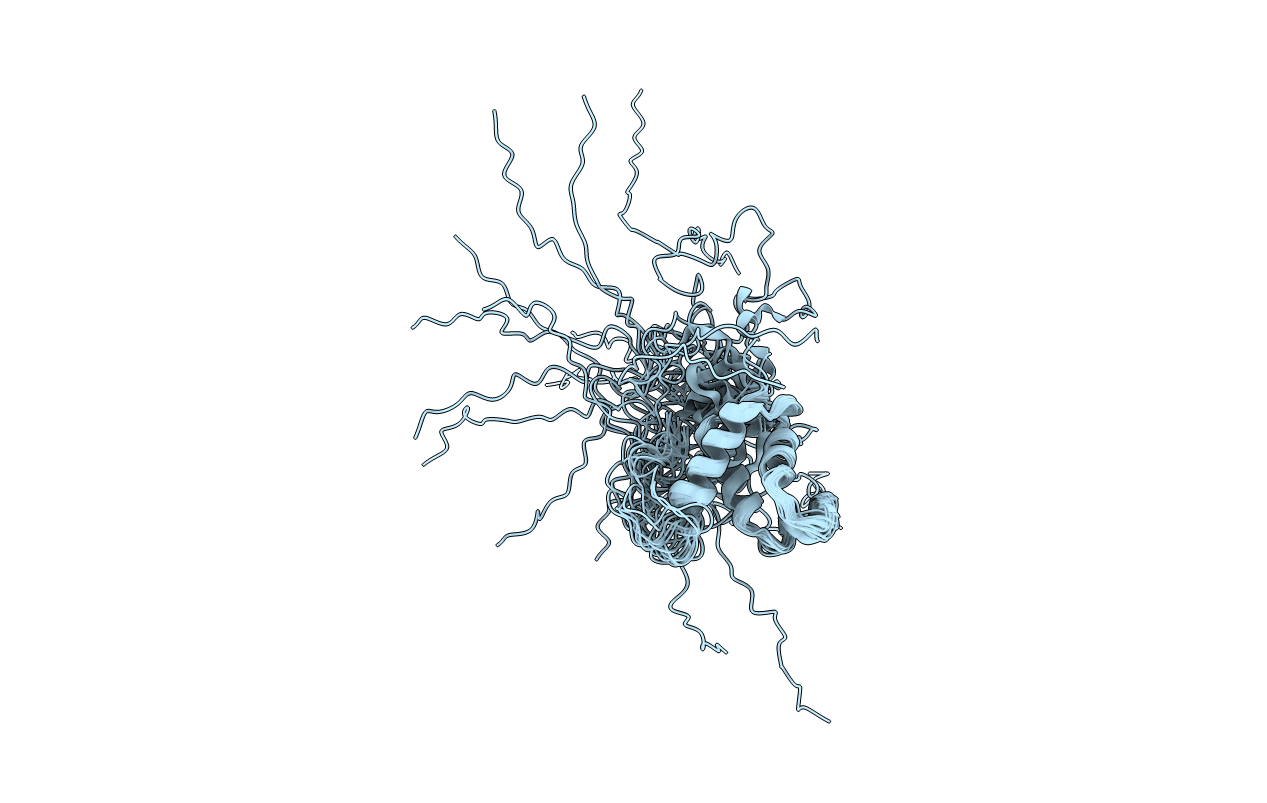
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
target function