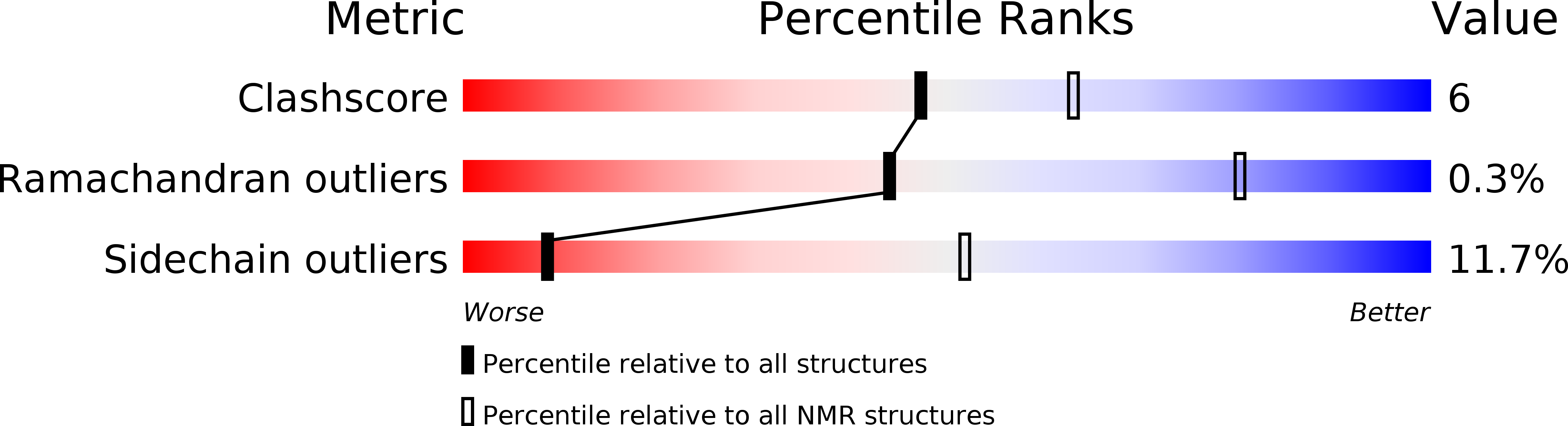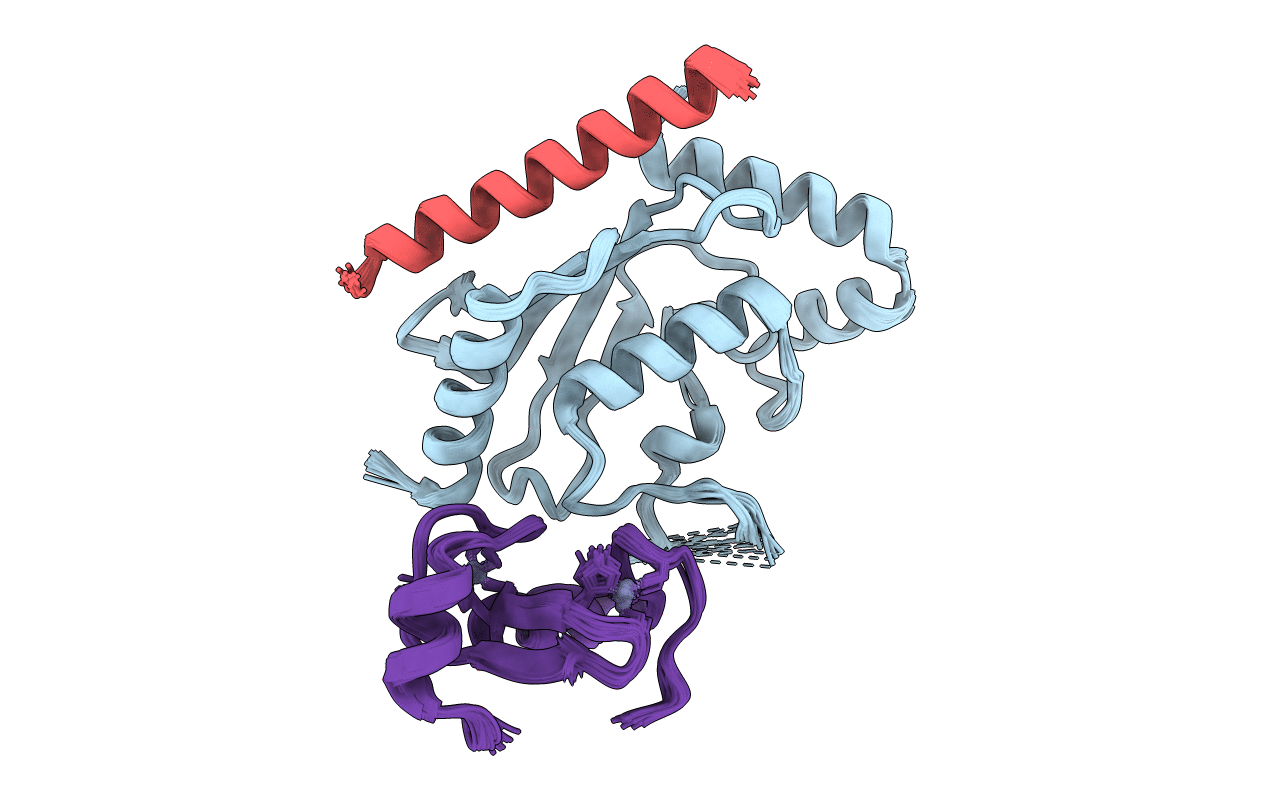
Deposition Date
2012-08-30
Release Date
2013-08-28
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2LXP
Keywords:
Title:
NMR structure of two domains in ubiquitin ligase gp78, RING and G2BR, bound to its conjugating enzyme Ube2g
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
Lowest Haddock Scores