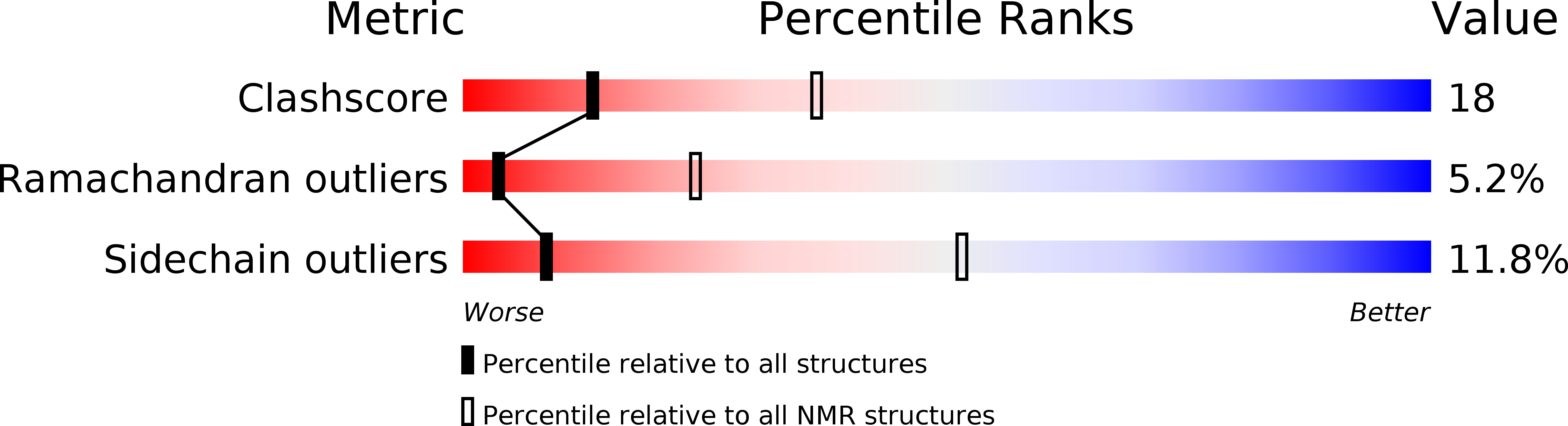
Deposition Date
2012-02-03
Release Date
2013-02-06
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2LP7
Keywords:
Title:
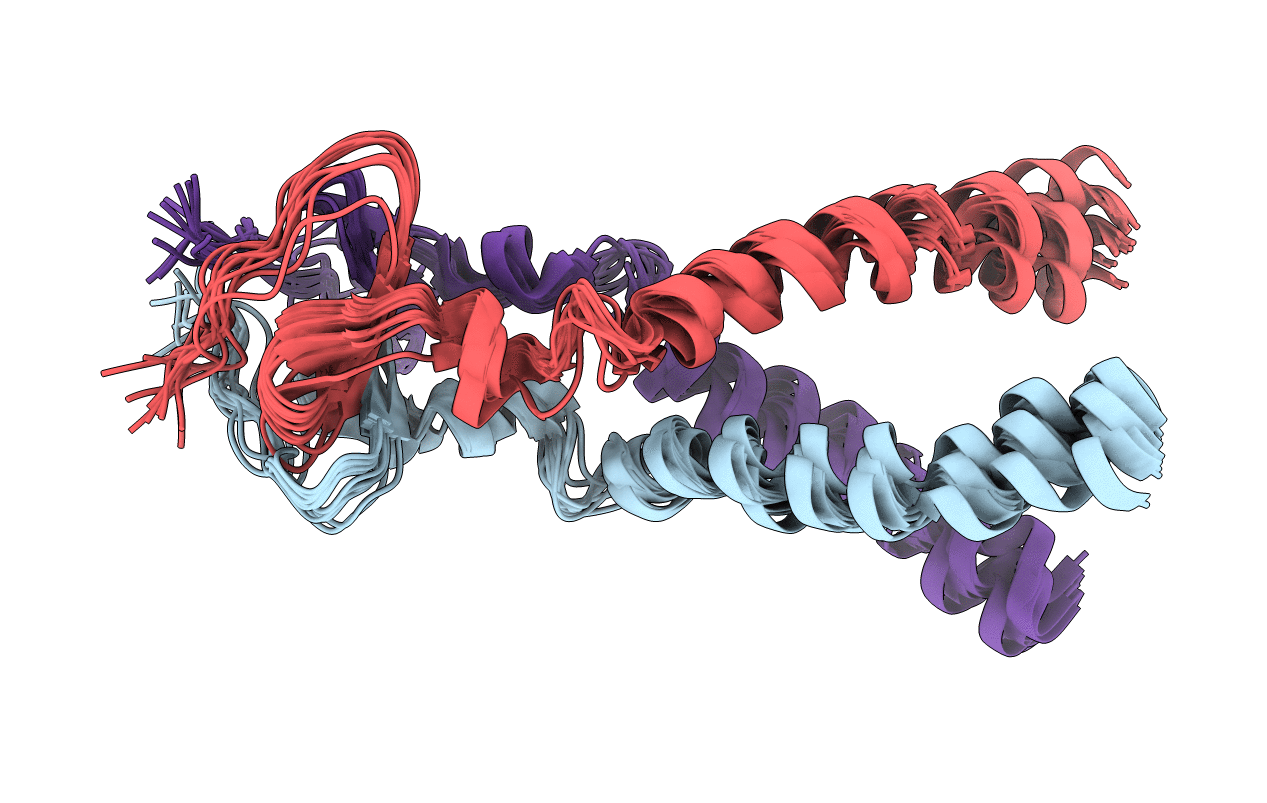
Structure of gp41-M-MAT, a membrane associated MPER trimer from HIV-1 gp41.
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
400
Conformers Submitted:
11
Selection Criteria:
structures with the least restraint violations