Deposition Date
2012-01-17
Release Date
2012-12-26
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2LO6
Keywords:
Title:
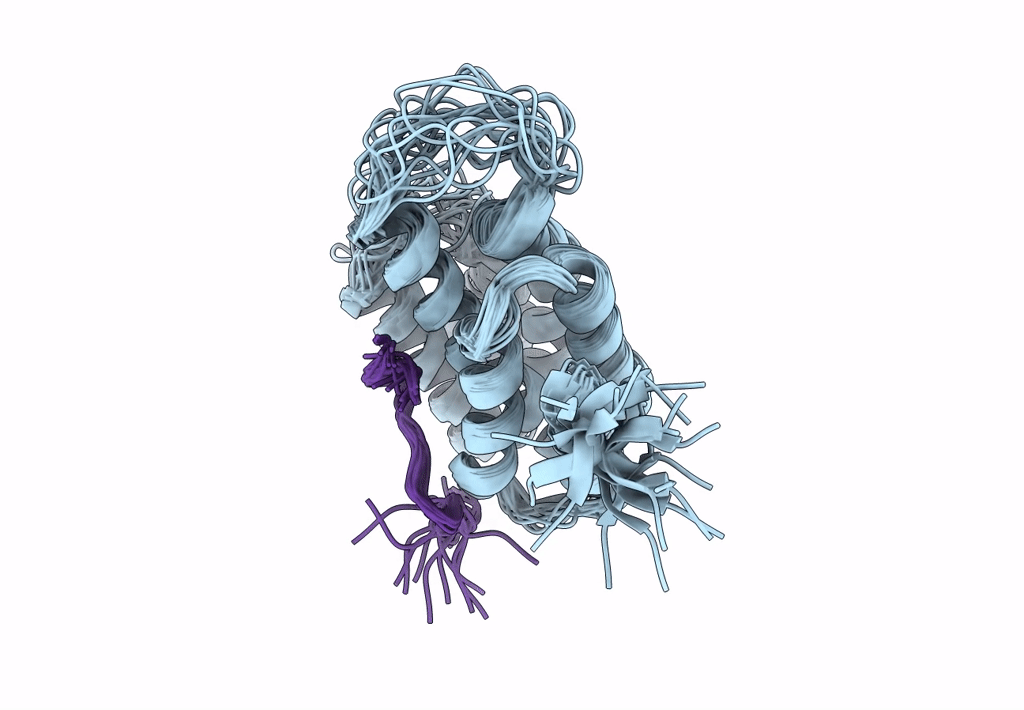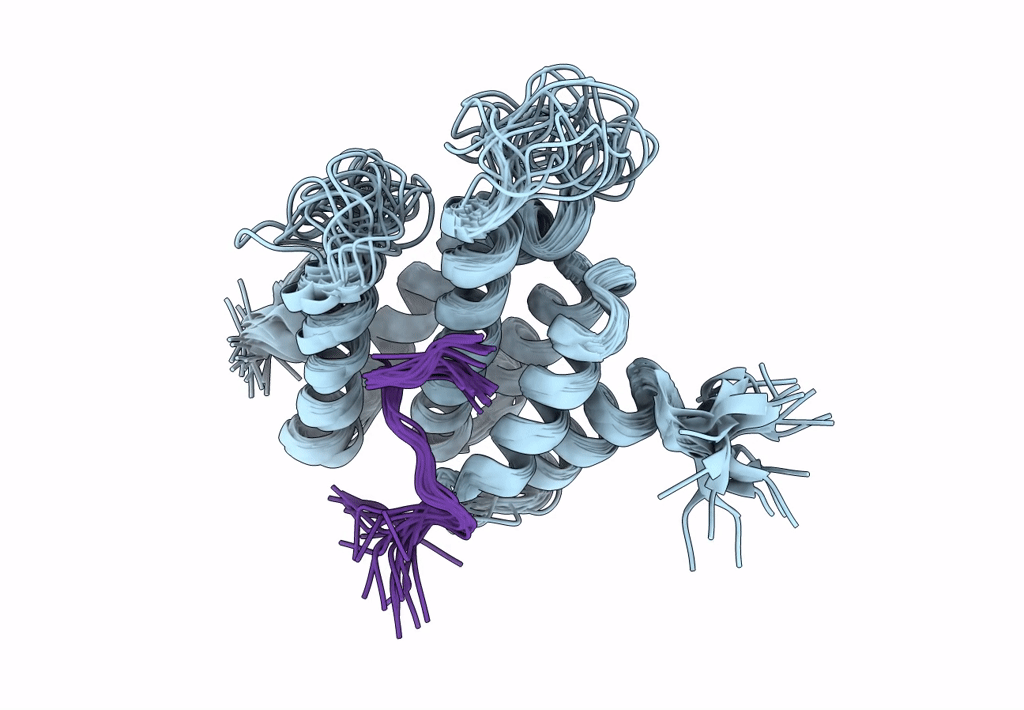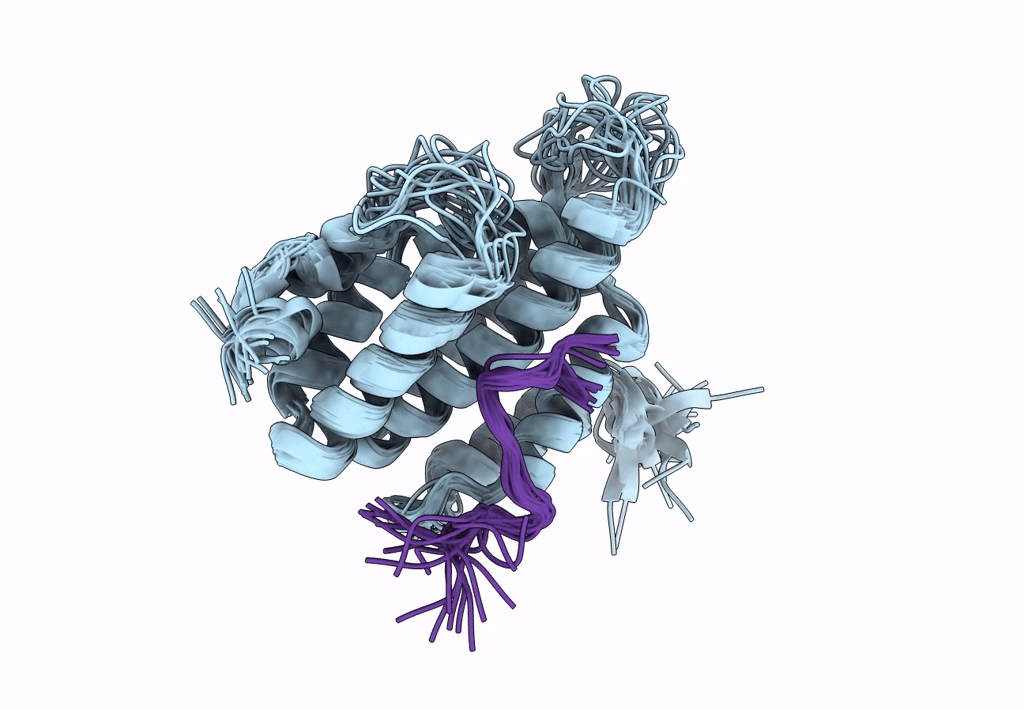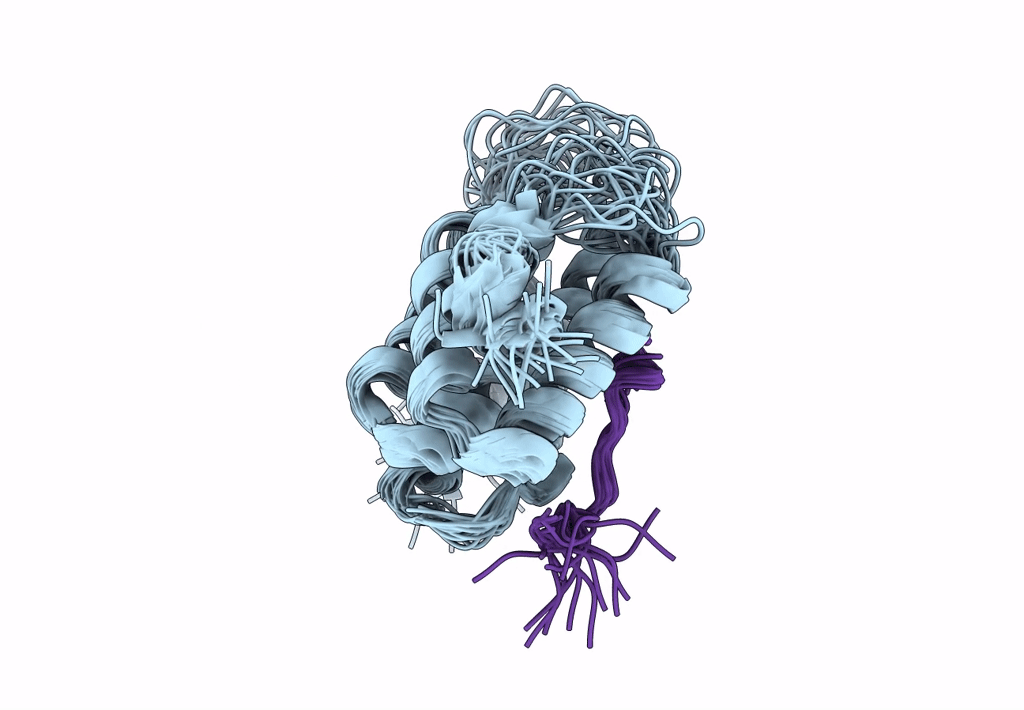
Structure of Nrd1 CID bound to phosphorylated RNAP II CTD
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae S288c (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


