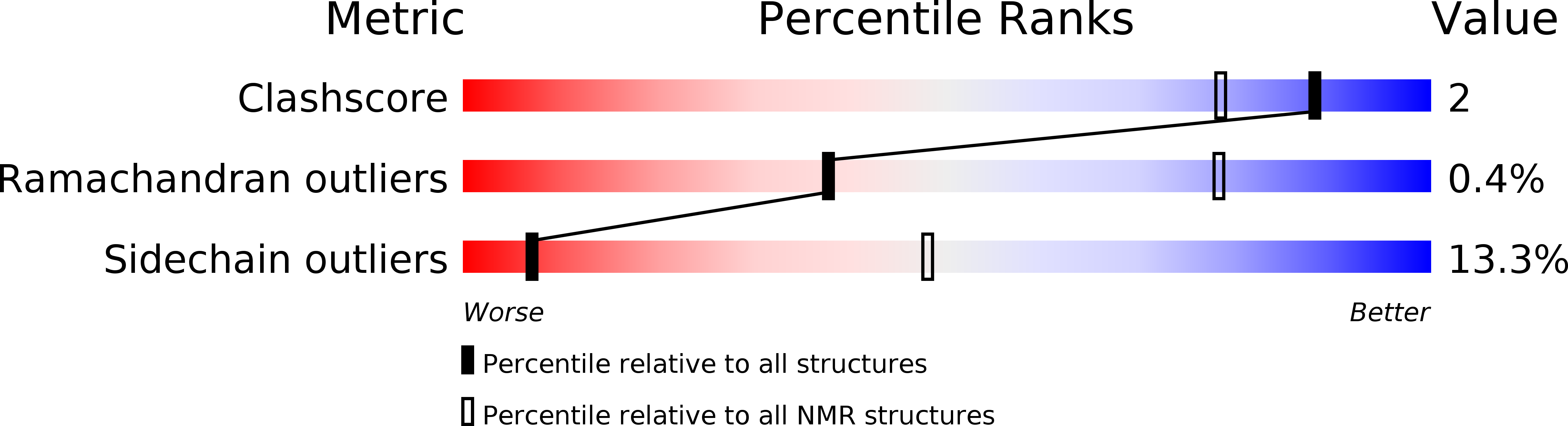
Deposition Date
2011-12-16
Release Date
2012-11-28
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2LN4
Keywords:
Title:
Insight into the antimicrobial activities based on the Structure-activity relationships of coprisin isolated from the Dung Beetle, Copris tripartitus
Biological Source:
Source Organism(s):
Copris tripartitus (Taxon ID: 438892)
Method Details:
Experimental Method:
Conformers Calculated:
500
Conformers Submitted:
20
Selection Criteria:
target function