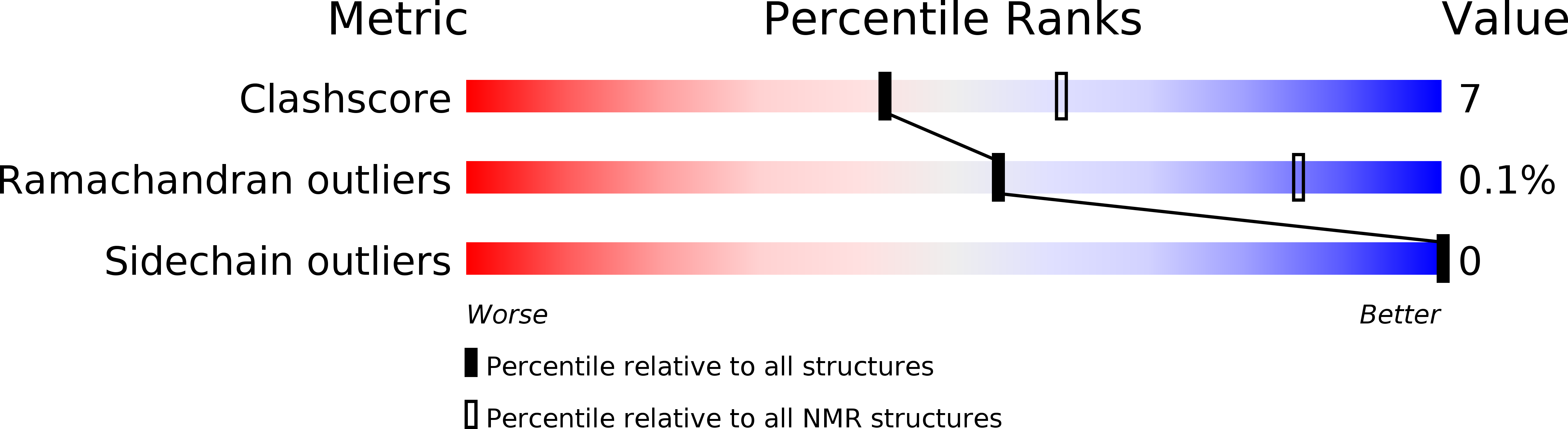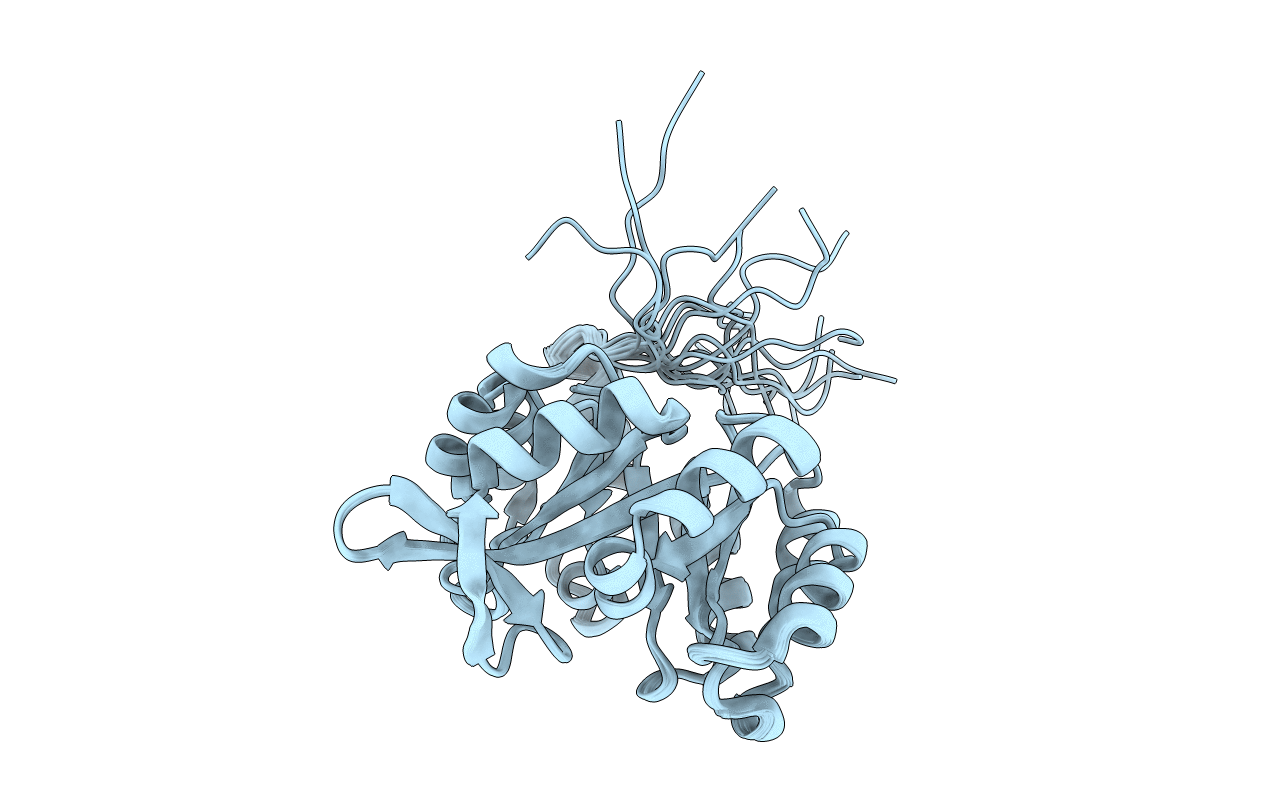
Deposition Date
2011-11-07
Release Date
2012-03-21
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2LLE
Keywords:
Title:
Computational design of an eight-stranded (beta/alpha)-barrel from fragments of different folds
Biological Source:
Source Organism(s):
Thermotoga maritima (Taxon ID: 2336)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
17
Selection Criteria:
structures with the least restraint violations