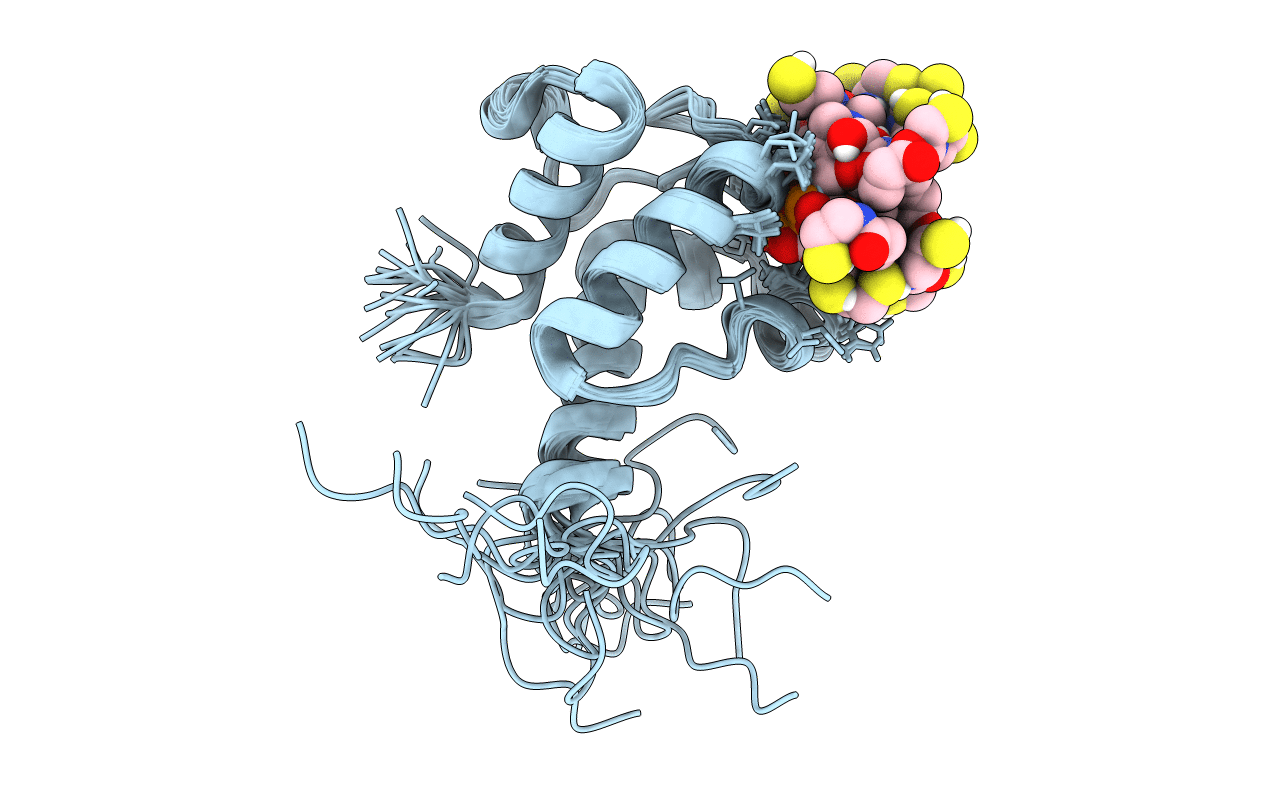
Deposition Date
2011-10-31
Release Date
2011-11-30
Last Version Date
2023-06-14
Entry Detail
PDB ID:
2LL8
Keywords:
Title:
Solution NMR structure of the specialized holo-acyl carrier protein RPA2022 from Rhodopseudomonas palustris refined with NH RDCs, Northeast Structural Genomics Consortium Target RpR324
Biological Source:
Source Organism(s):
Rhodopseudomonas palustris (Taxon ID: 1076)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


