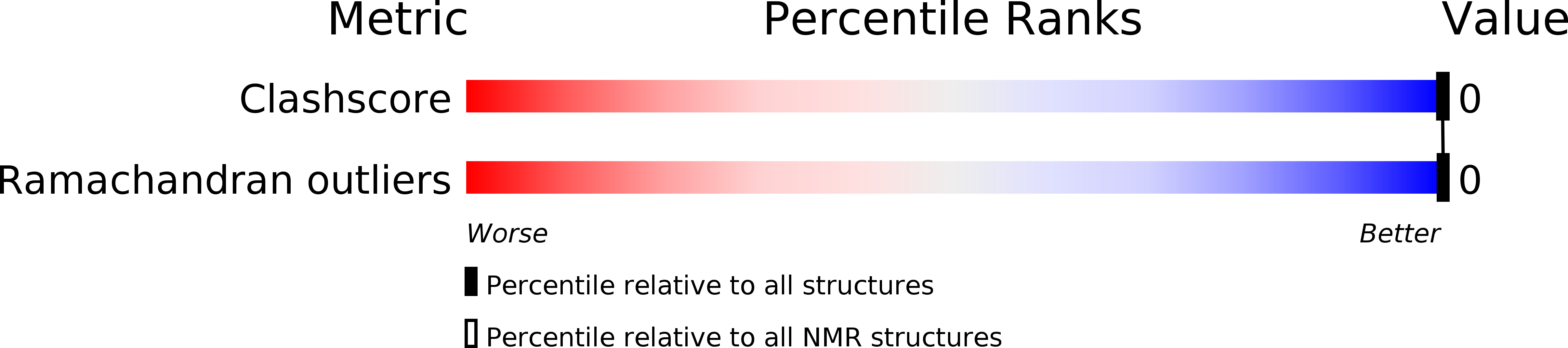
Deposition Date
2011-10-07
Release Date
2011-11-09
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2LK9
Keywords:
Title:
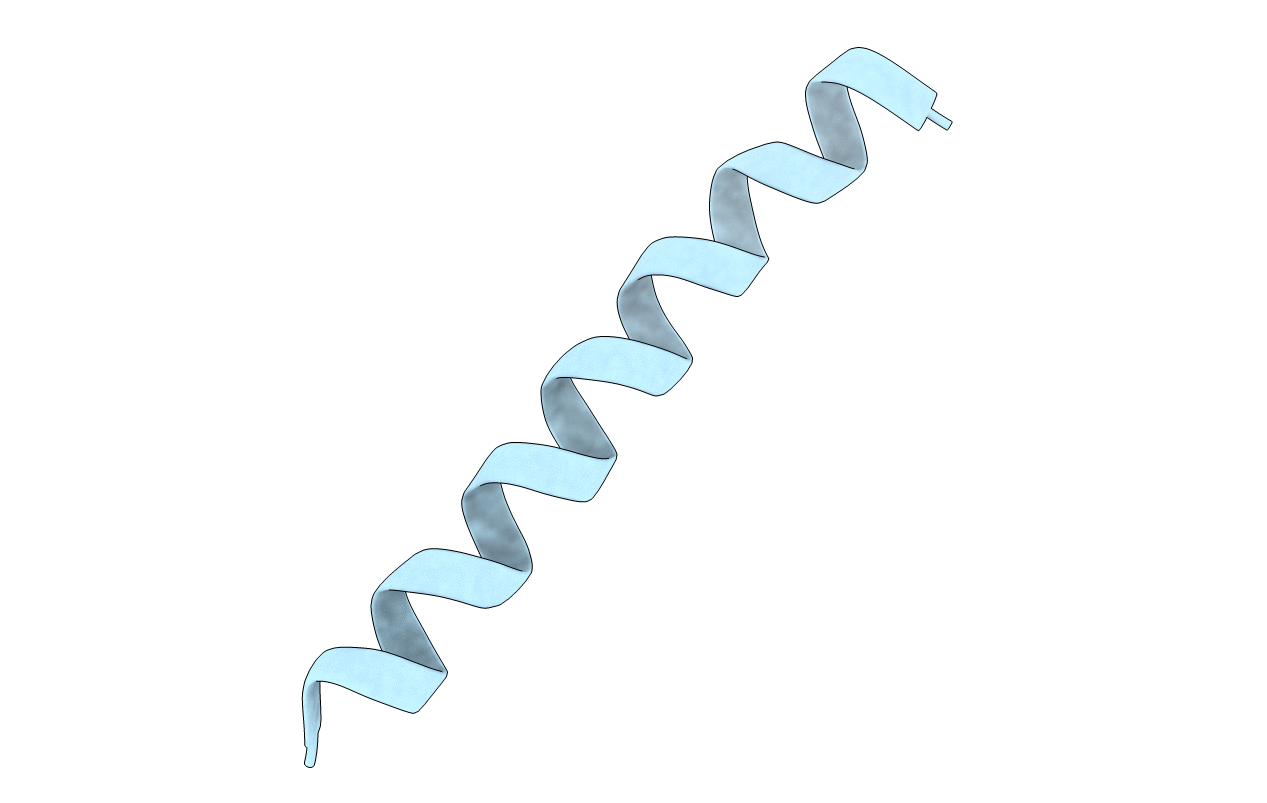
Structure of BST-2/Tetherin Transmembrane Domain
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
1
Selection Criteria:
structures with the lowest energy