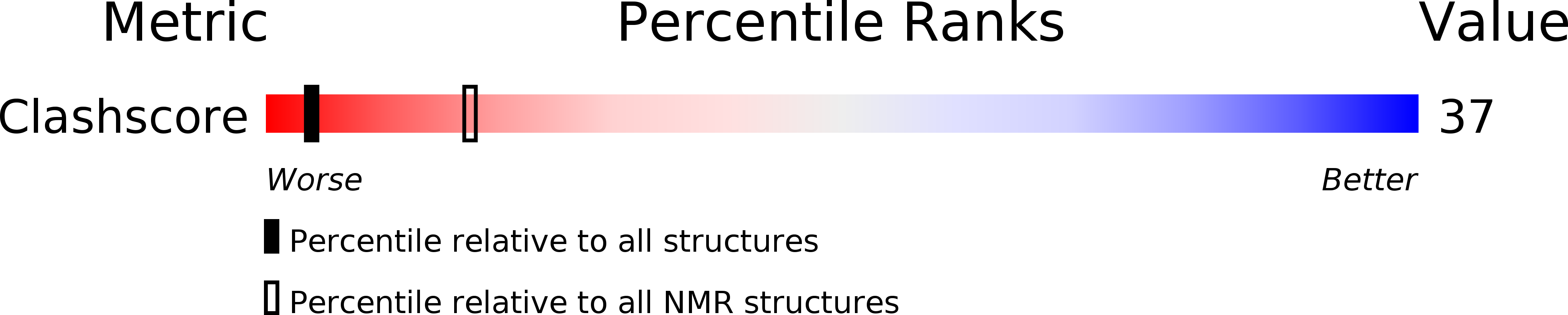
Deposition Date
2011-04-07
Release Date
2012-02-22
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2LBY
Keywords:
Title:
G-quadruplex structure formed at the 5'-end of NHEIII_1 element in human c-MYC promoter
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
10
Selection Criteria:
structures with the least restraint violations