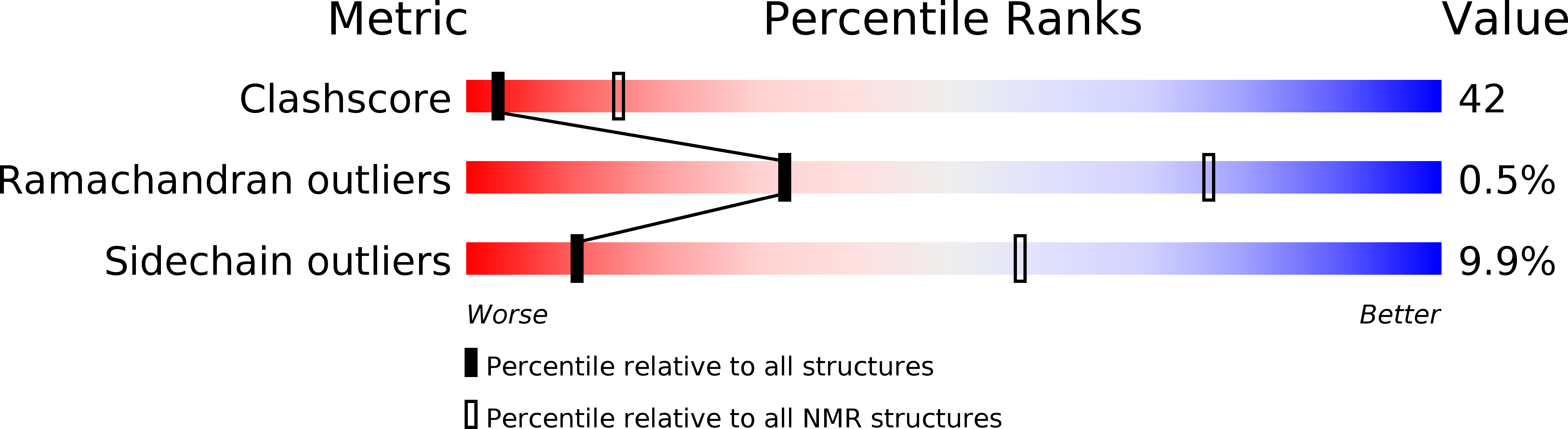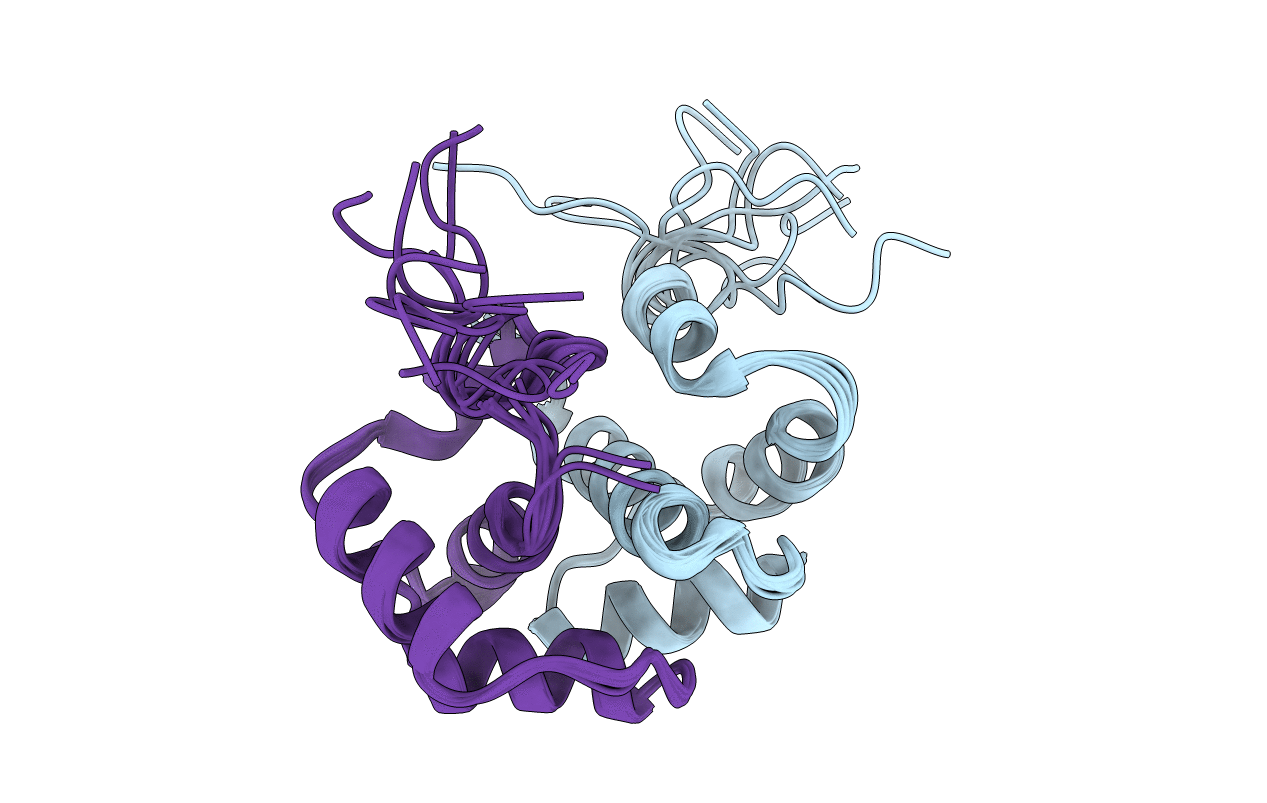
Deposition Date
2011-03-30
Release Date
2011-12-14
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2LBF
Keywords:
Title:
Solution structure of the dimerization domain of human ribosomal protein P1/P2 heterodimer
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the least restraint violations