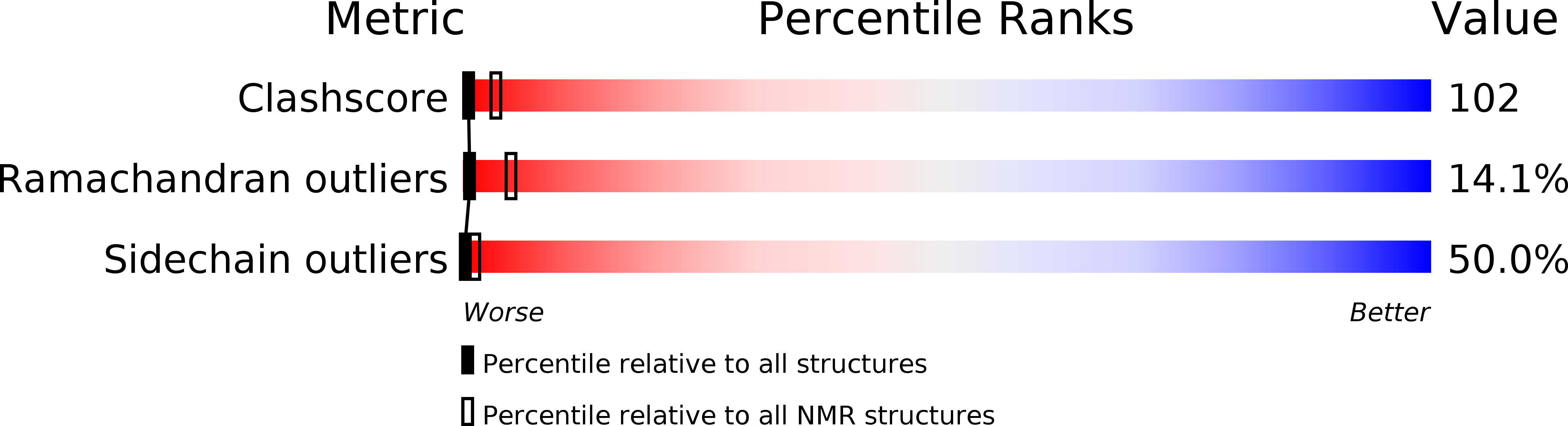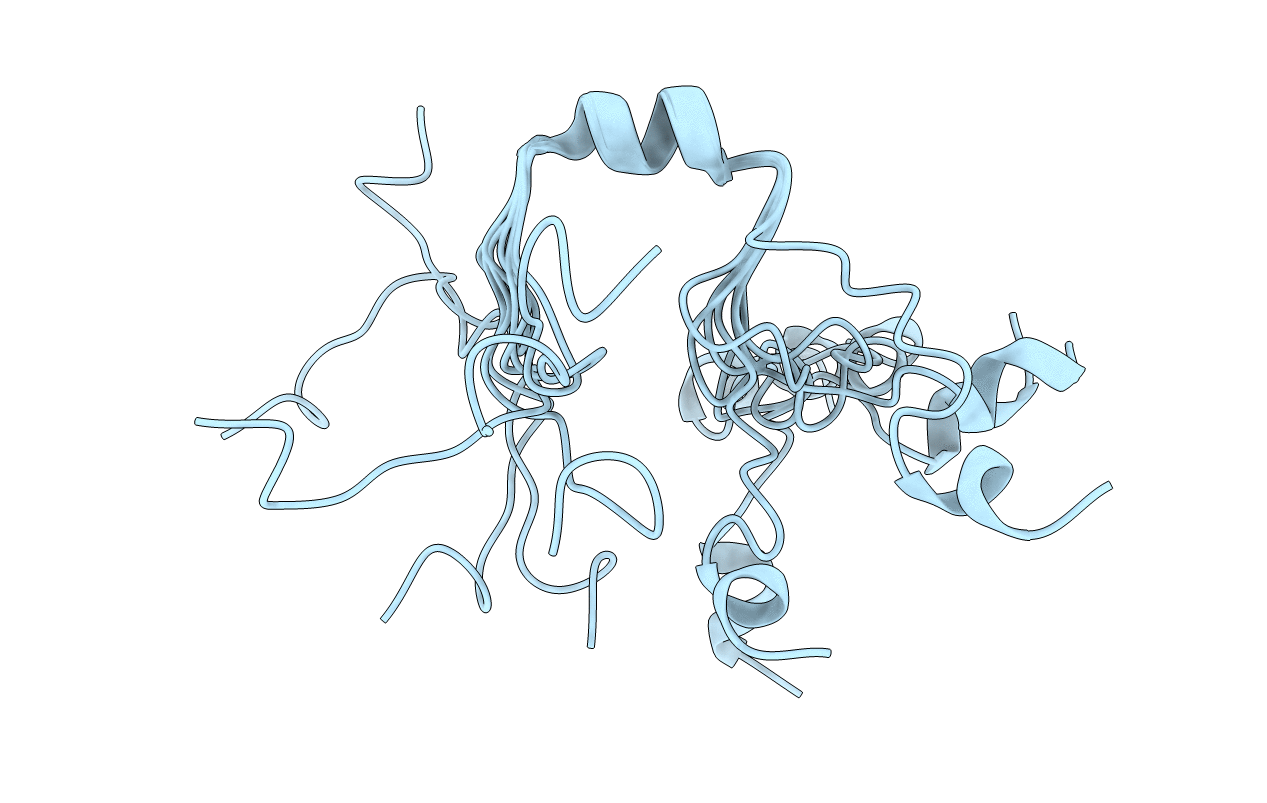
Deposition Date
2010-09-13
Release Date
2010-10-06
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2L3H
Keywords:
Title:
NMR Structure in a Membrane Environment Reveals Putative Amyloidogenic Regions of the SEVI Precursor Peptide PAP248-286
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
8
Selection Criteria:
structures with the least restraint violations