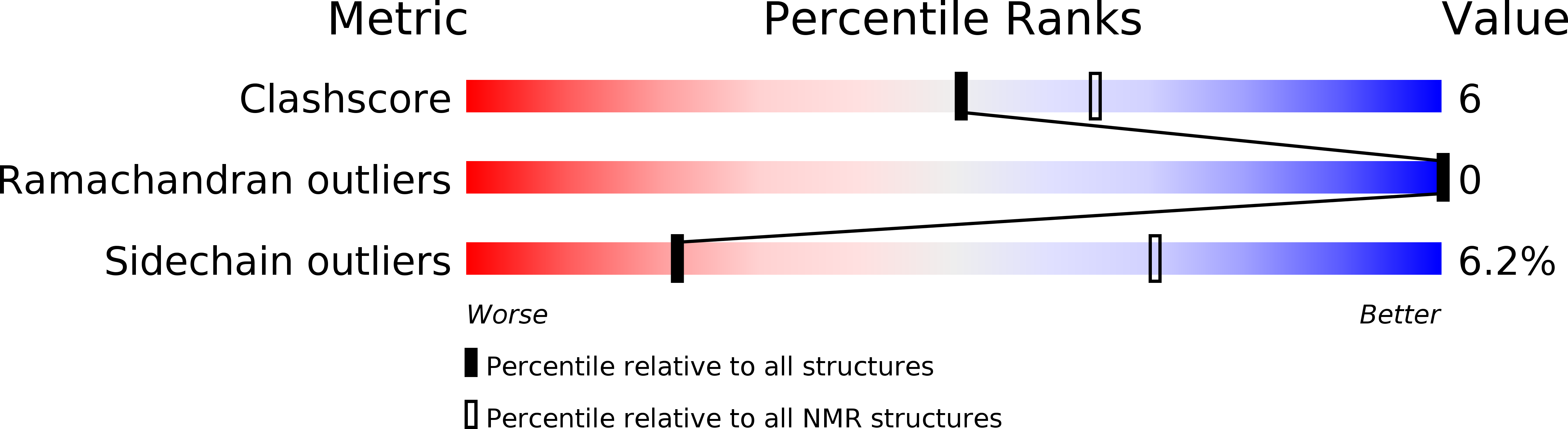Deposition Date
2010-09-08
Release Date
2011-01-19
Last Version Date
2024-10-30
Entry Detail
PDB ID:
2L37
Keywords:
Title:
3D solution structure of arginine/glutamate-rich polypeptide Luffin P1 from the seeds of sponge gourd (Luffa cylindrical)
Biological Source:
Source Organism(s):
Luffa aegyptiaca (Taxon ID: 3670)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
target function