Deposition Date
2010-08-27
Release Date
2011-07-13
Last Version Date
2024-05-01
Entry Detail
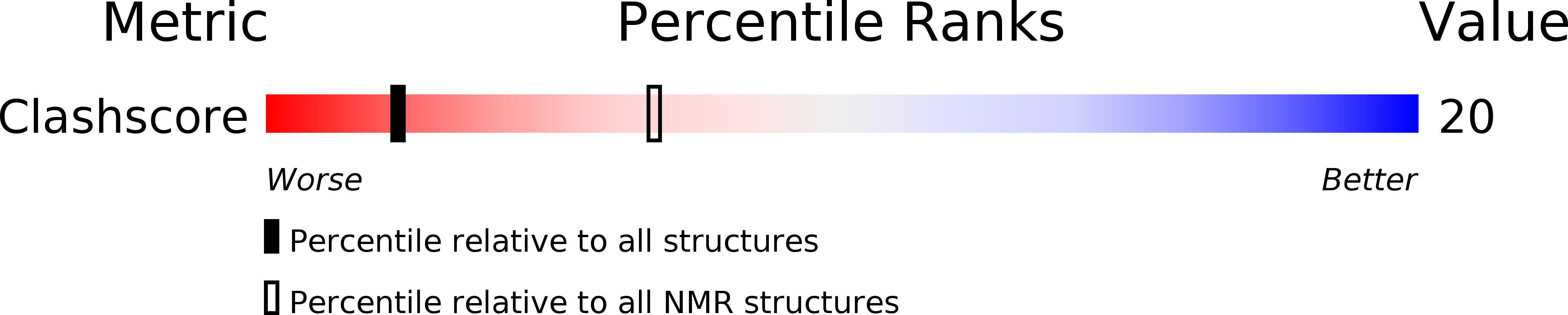
PDB ID:
2L2V
Keywords:
Title:
NMR Solution Structures of -3 (3' staggered) Bistranded Abasic Site Lesions in DNA
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
4
Selection Criteria:
structures with the least restraint violations