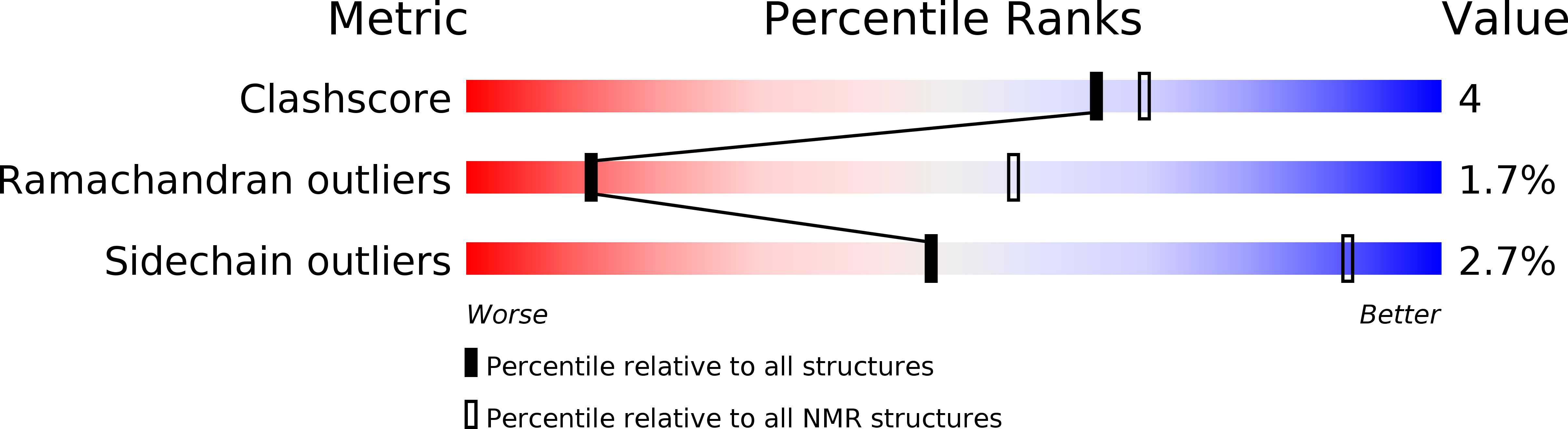
Deposition Date
2010-05-27
Release Date
2010-06-09
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2KYI
Keywords:
Title:
Solution NMR structure of Dsy0195(21-82) protein from Desulfitobacterium Hafniense. Northeast Structural Genomics Consortium Target DhR8C
Biological Source:
Source Organism(s):
Desulfitobacterium hafniense (Taxon ID: 138119)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
150
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy