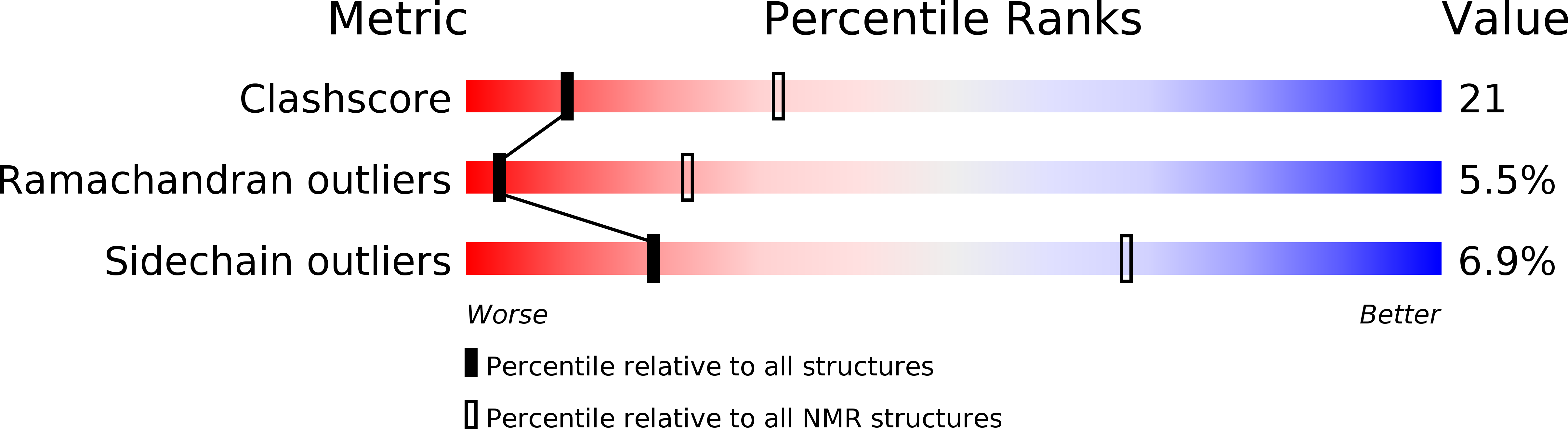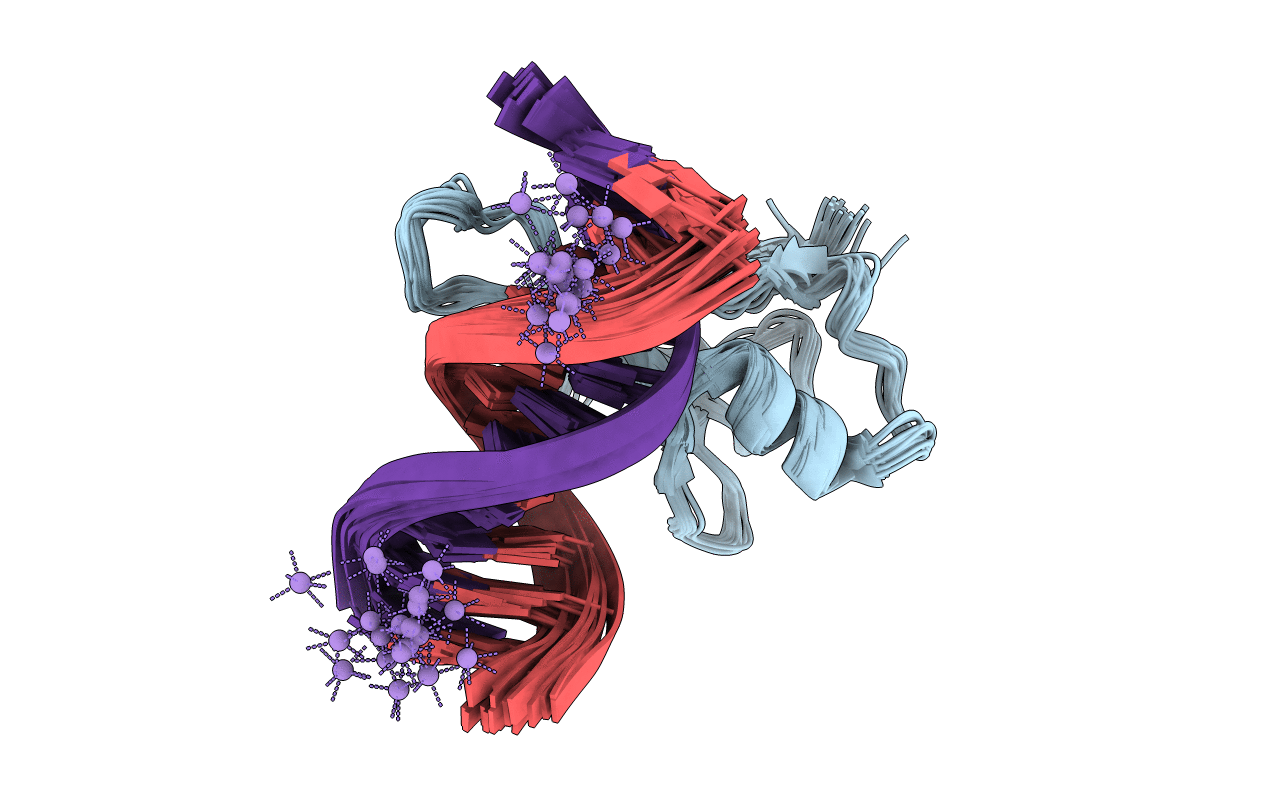
Deposition Date
2010-05-18
Release Date
2011-05-18
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2KY8
Keywords:
Title:
Solution structure and dynamic analysis of chicken MBD2 methyl binding domain bound to a target methylated DNA sequence
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
all calculated structures submitted