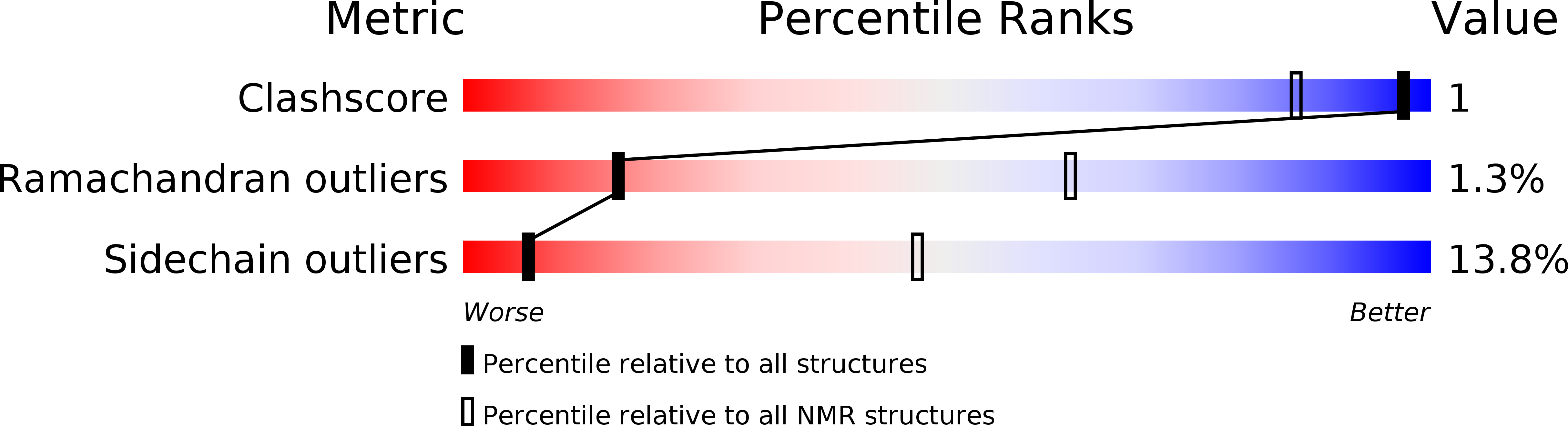
Deposition Date
2010-03-04
Release Date
2010-03-16
Last Version Date
2024-10-30
Entry Detail
PDB ID:
2KV1
Keywords:
Title:
Insights into Function, Catalytic Mechanism and Fold Evolution of Mouse Selenoprotein Methionine Sulfoxide Reductase B1 through Structural Analysis
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
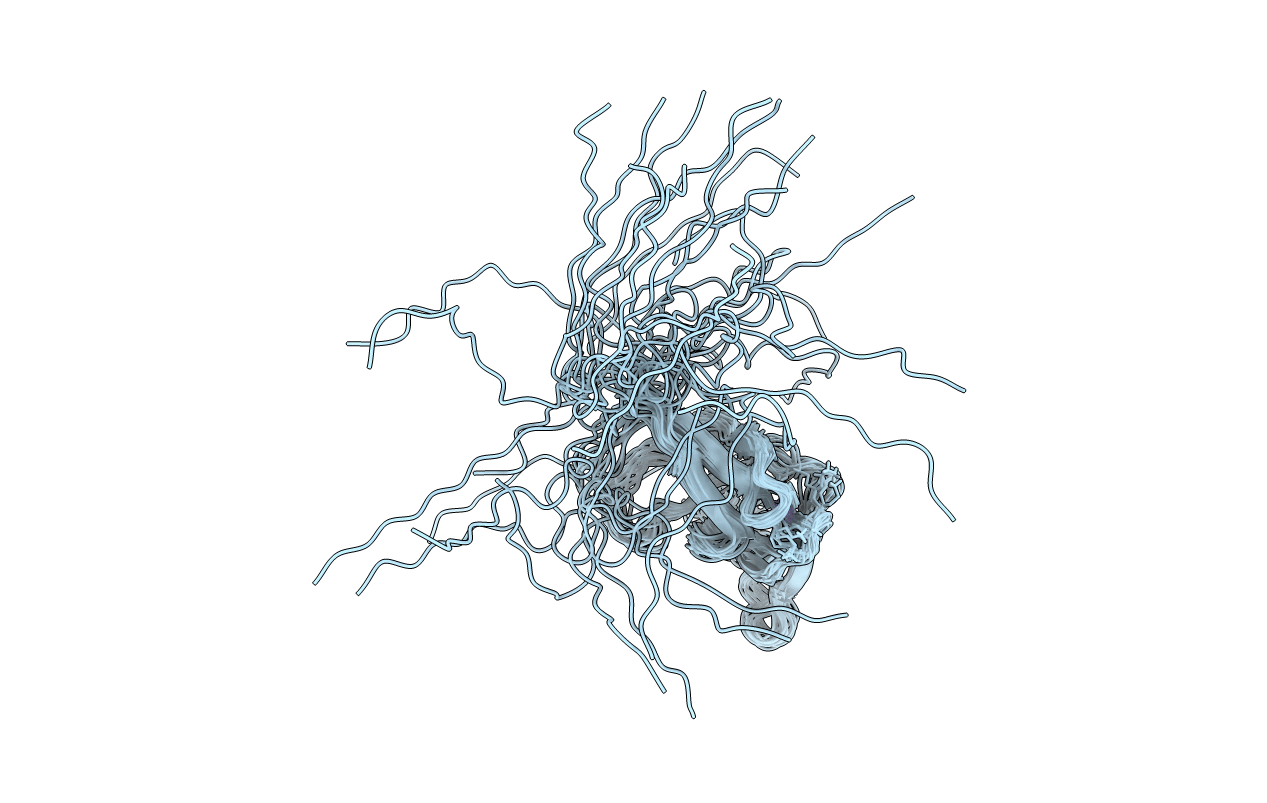
Conformers Calculated:
96
Conformers Submitted:
20
Selection Criteria:
target function