Deposition Date
2010-02-10
Release Date
2010-04-28
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2KU0
Keywords:
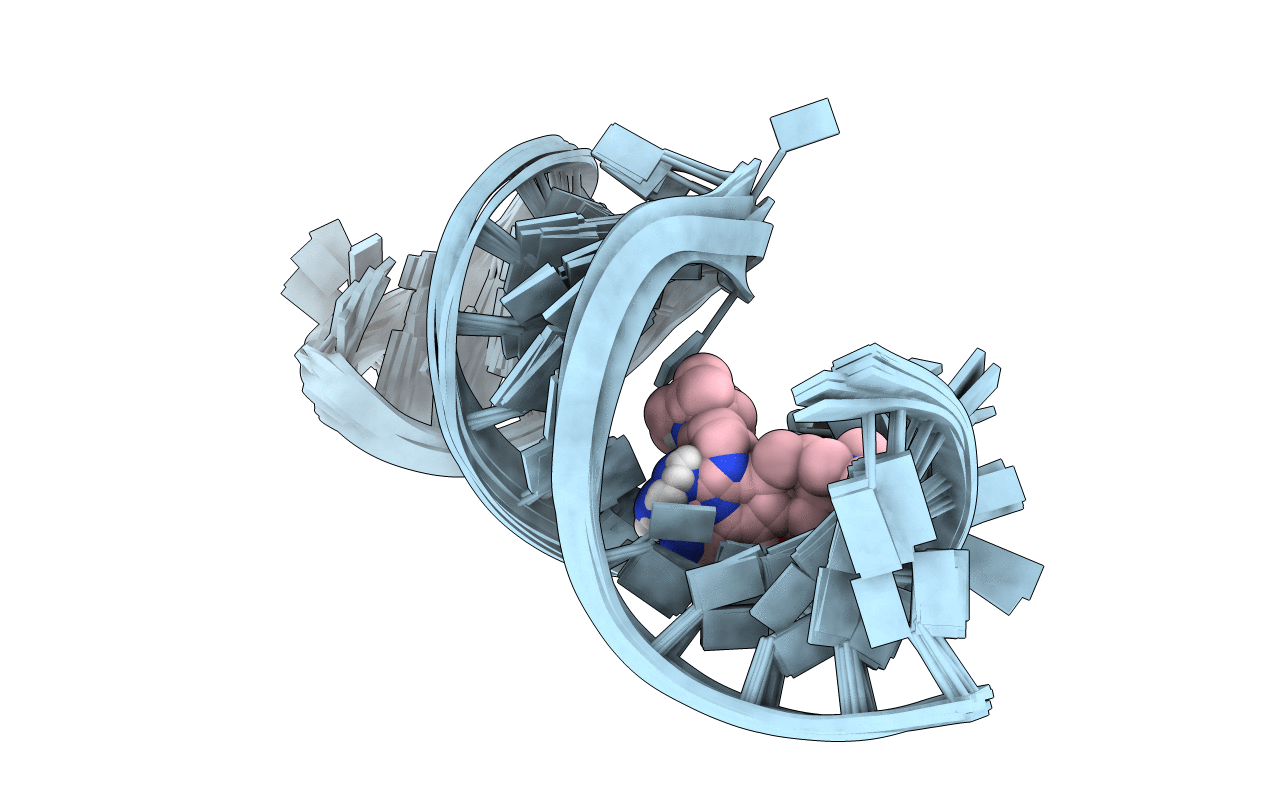
Title:
Inhibitor Induced Structural Change in the HCV IRES Domain IIa RNA
Biological Source:
Source Organism(s):
Hepatitis C virus (Taxon ID: 11103)
Method Details:
Experimental Method:
Conformers Calculated:
19
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy


