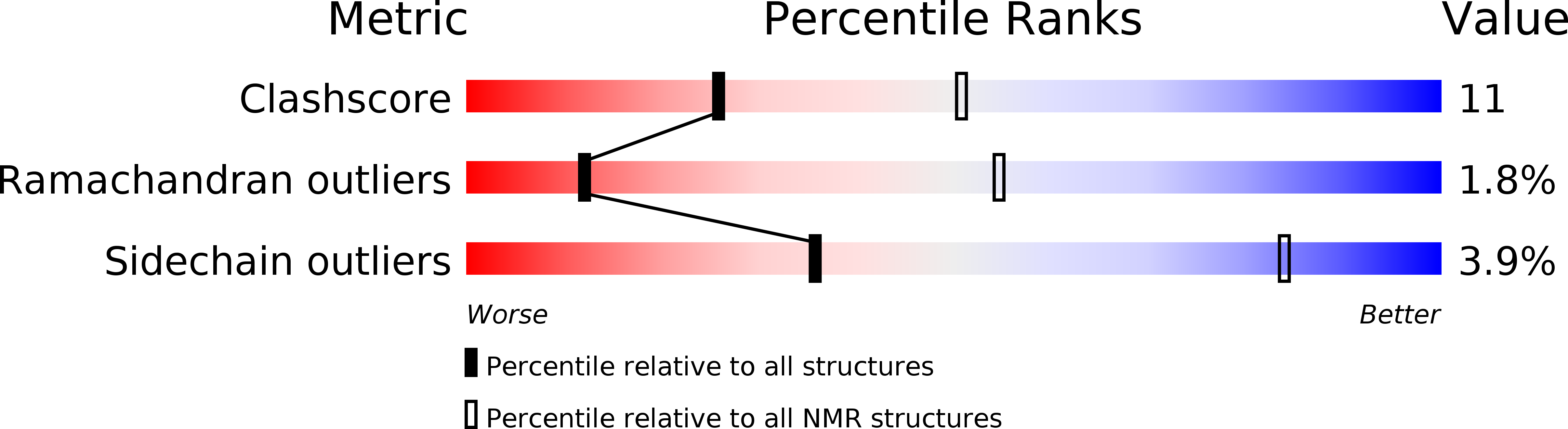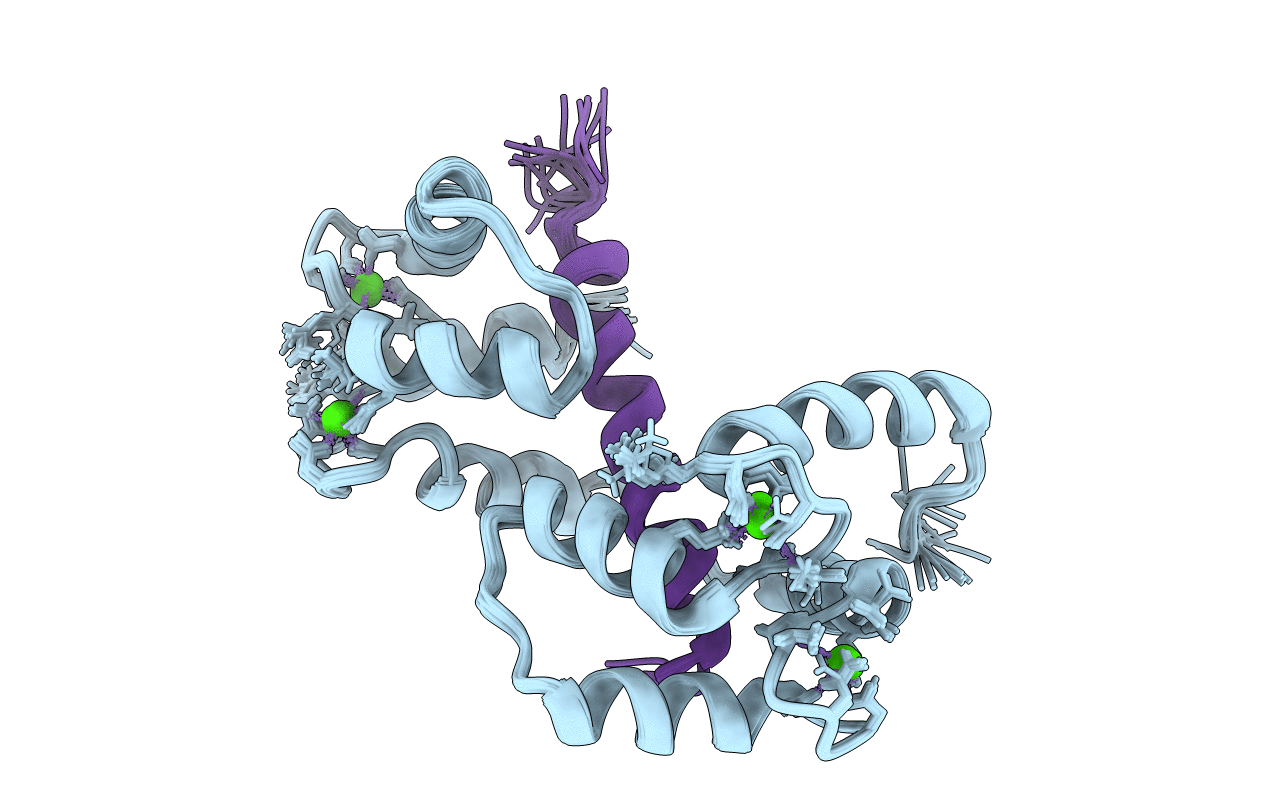
Deposition Date
2009-08-21
Release Date
2009-11-24
Last Version Date
2024-05-08
Entry Detail
PDB ID:
2KNE
Keywords:
Title:
Calmodulin wraps around its binding domain in the plasma membrane CA2+ pump anchored by a novel 18-1 motif
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy