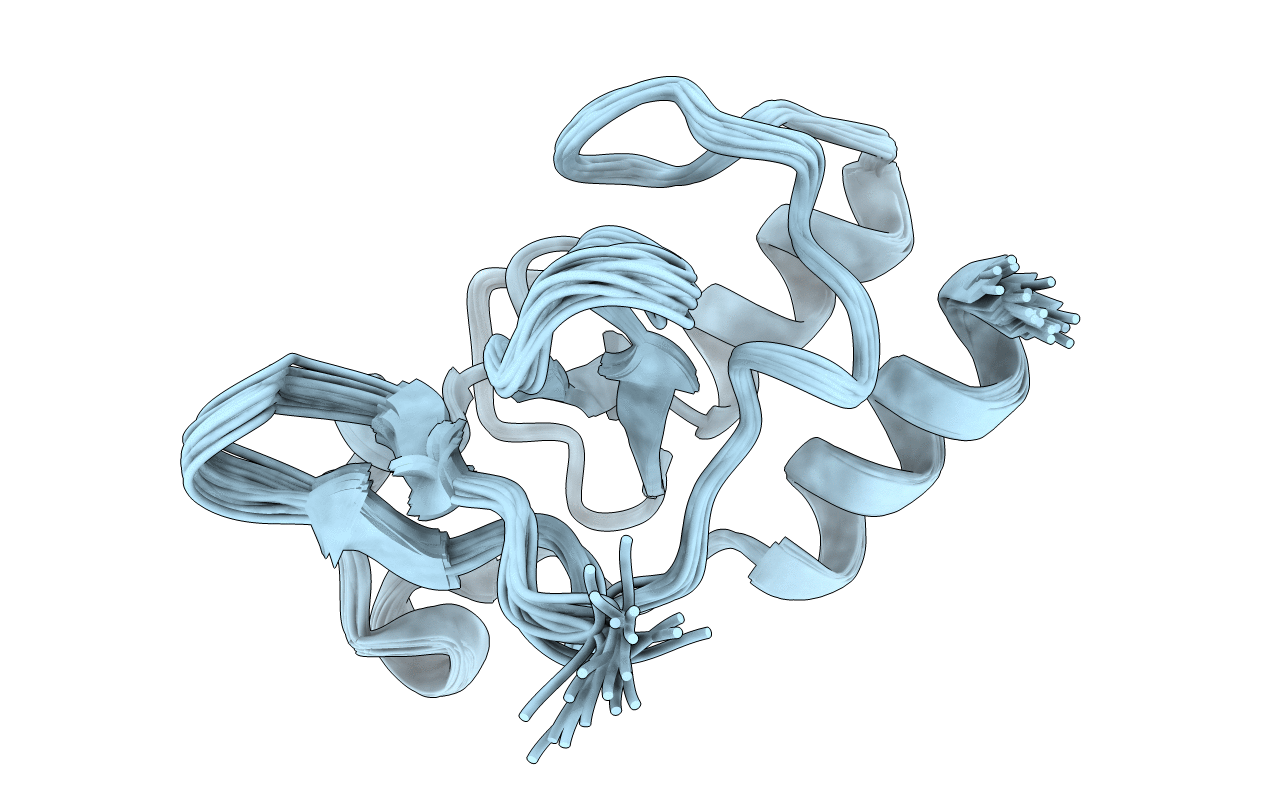
Deposition Date
2009-06-29
Release Date
2009-08-25
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2KKY
Keywords:
Title:
Solution Structure of C-terminal domain of oxidized NleG2-3 (residue 90-191) from Pathogenic E. coli O157:H7. Northeast Structural Genomics Consortium and Midwest Center for Structural Genomics target ET109A
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 83334)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


