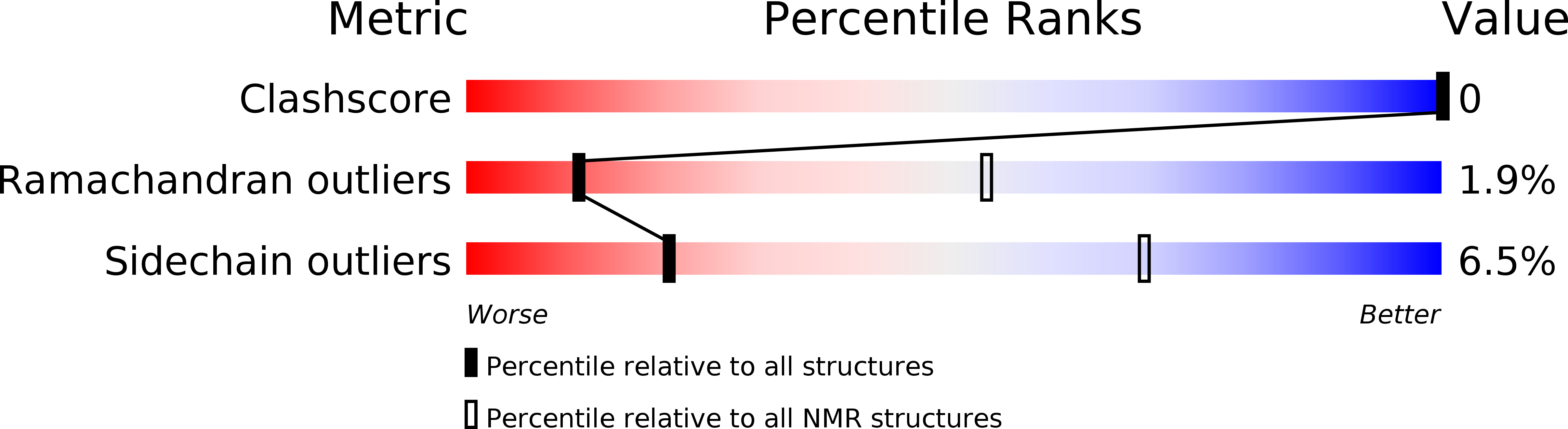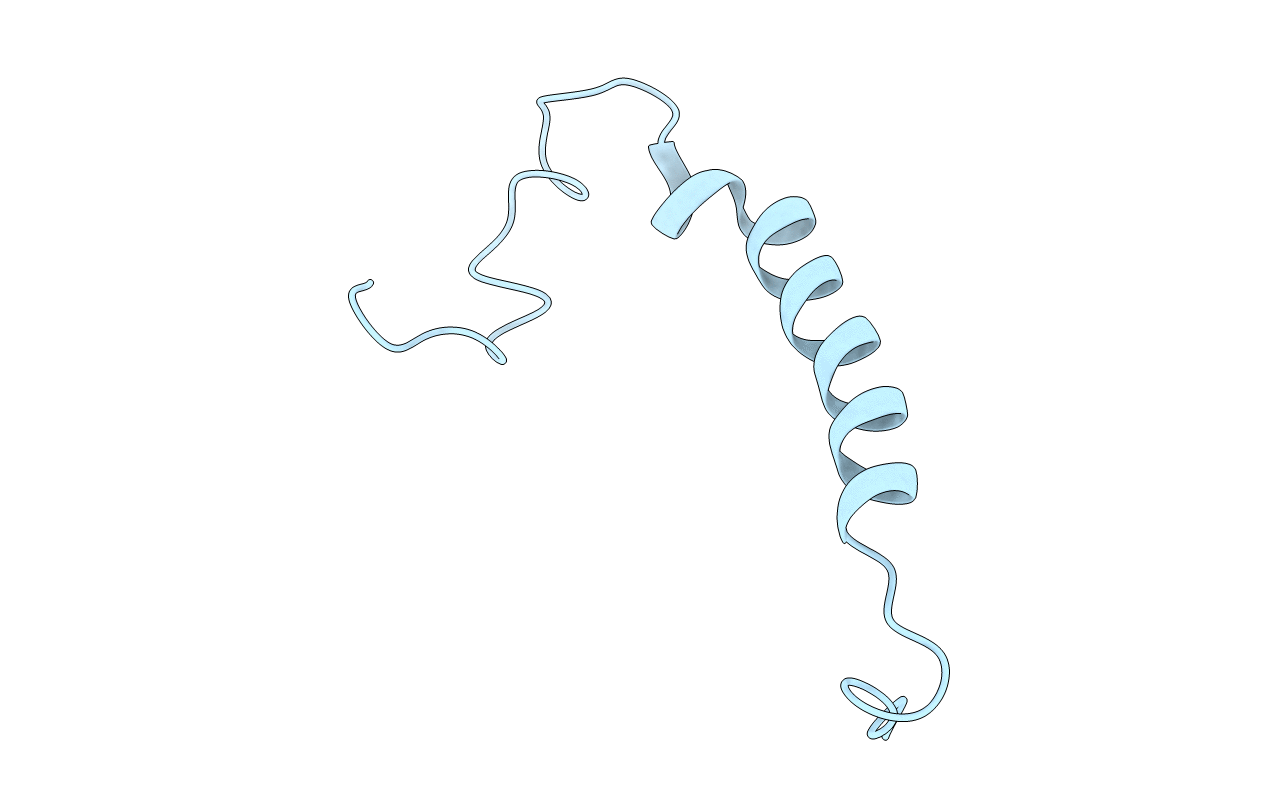
Deposition Date
2009-06-16
Release Date
2010-06-16
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2KK9
Keywords:
Title:
Anti-group A streptococcal vaccine epitope: structure, stability and its ability to interact with HLA class II molecules
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
1
Selection Criteria:
structures with the lowest energy