Deposition Date
2009-03-18
Release Date
2010-03-09
Last Version Date
2024-05-01
Entry Detail
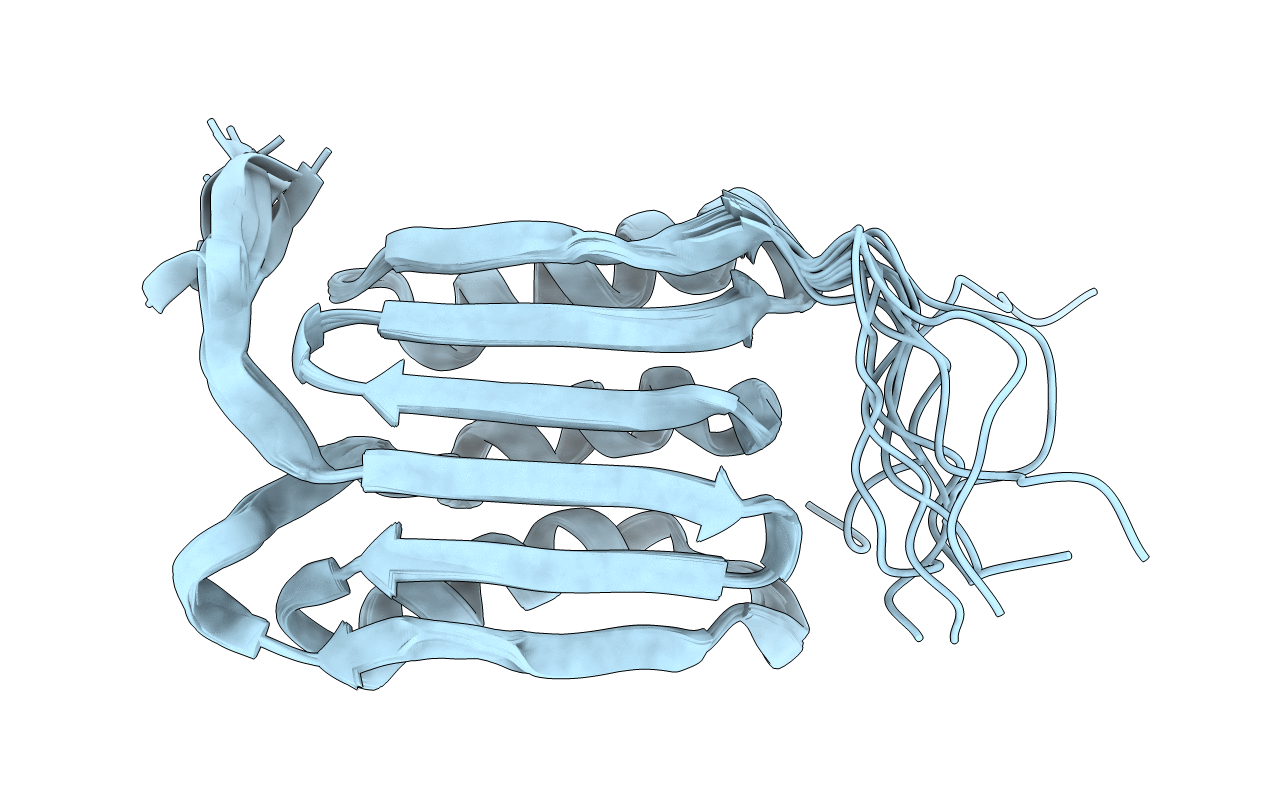
PDB ID:
2KGS
Keywords:
Title:
Solution structure of the amino-terminal domain of OmpATb, a pore forming protein from Mycobacterium tuberculosis
Biological Source:
Source Organism(s):
Mycobacterium tuberculosis (Taxon ID: 1773)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
10
Selection Criteria:
target function


