Deposition Date
2009-01-12
Release Date
2009-12-29
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2KDM
Keywords:
Title:
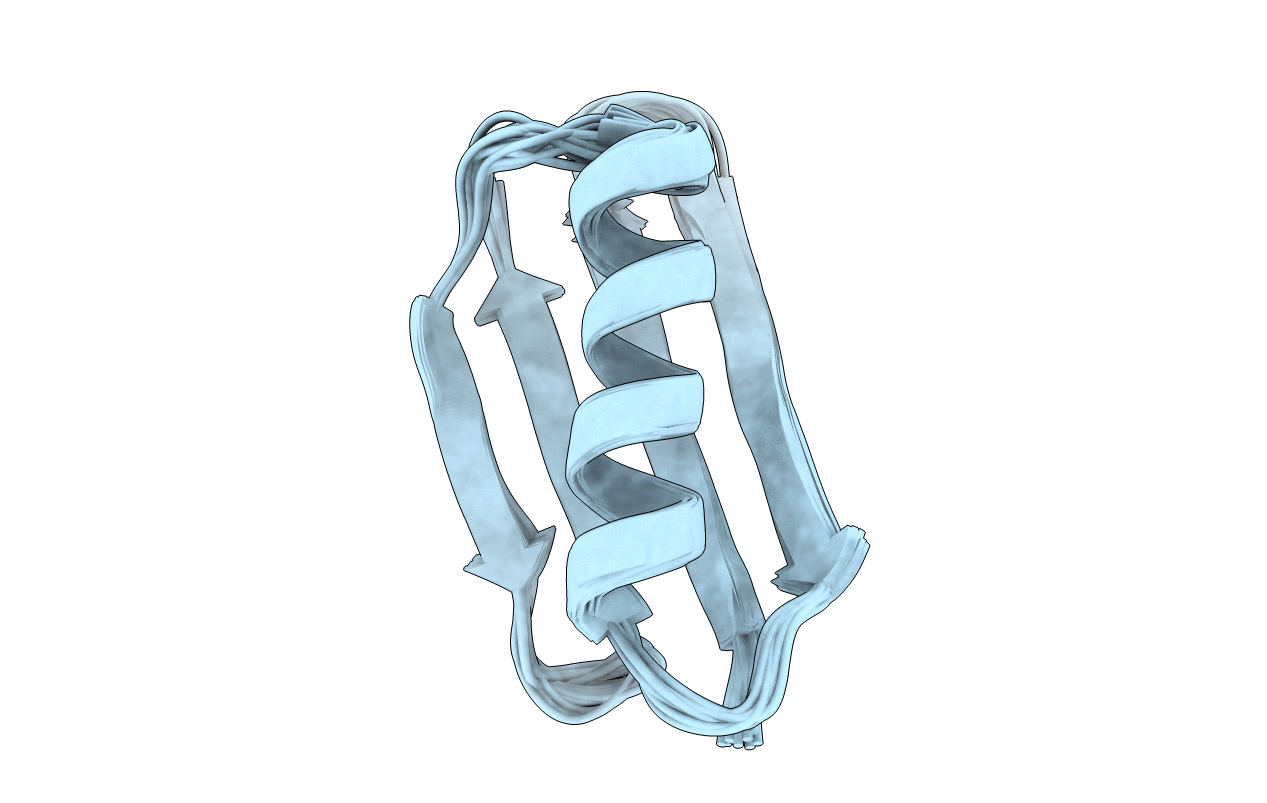
NMR structures of GA95 and GB95, two designed proteins with 95% sequence identity but different folds and functions
Method Details:
Experimental Method:
Conformers Calculated:
300
Conformers Submitted:
20
Selection Criteria:
structures with acceptable covalent geometry


