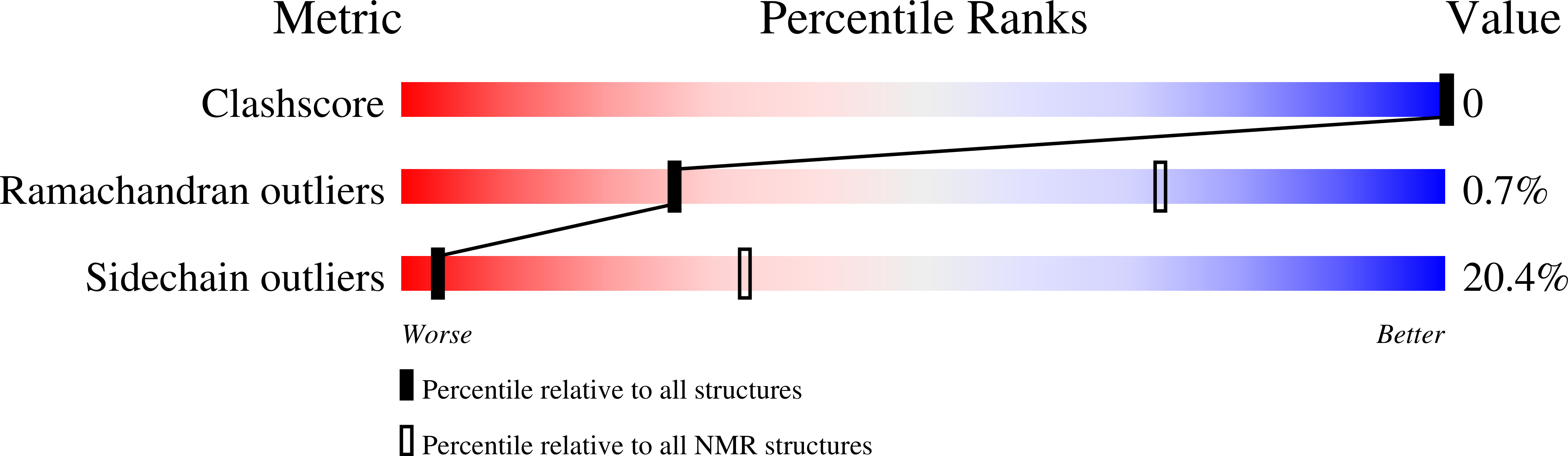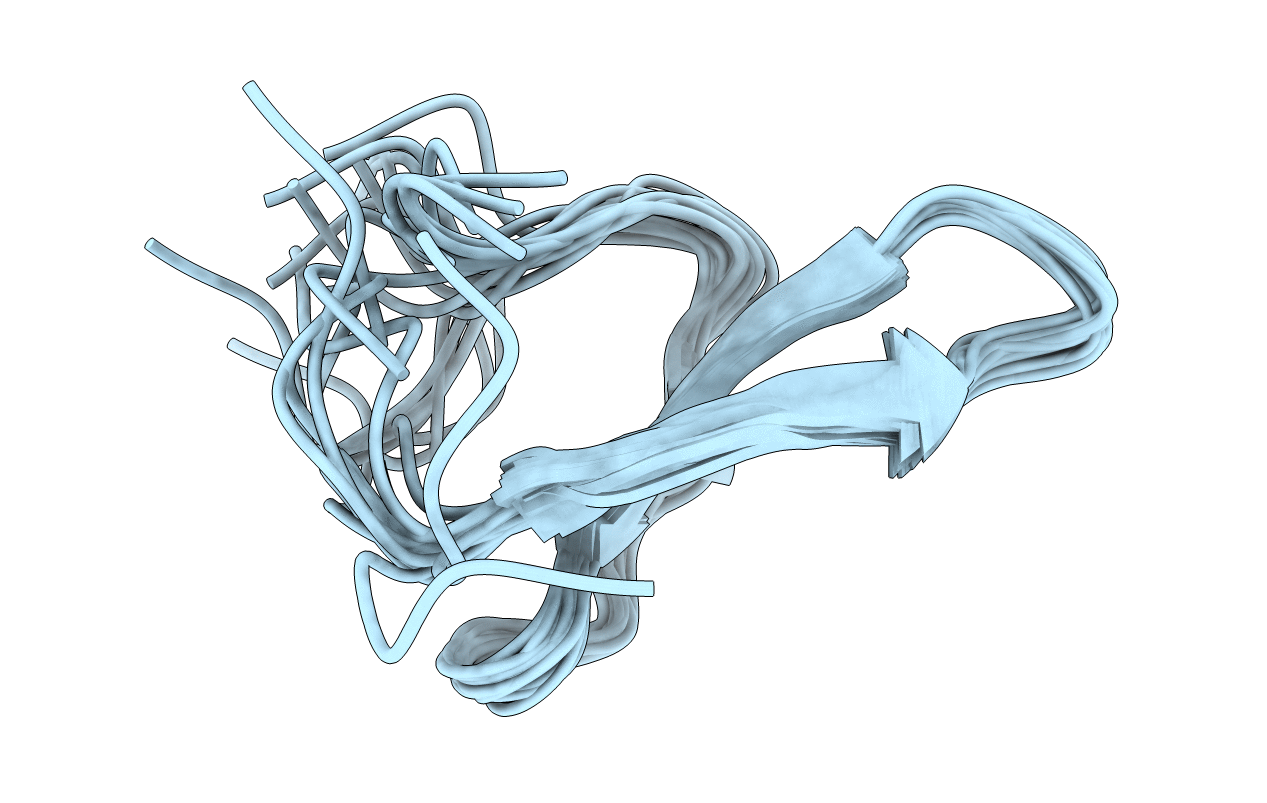
Deposition Date
2008-12-08
Release Date
2009-07-07
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2KBU
Keywords:
Title:
NMR solution structure of Pin1 WW domain mutant with beta turn mimic at position 12
Method Details:
Experimental Method:
Conformers Calculated:
27
Conformers Submitted:
15
Selection Criteria:
structures with acceptable covalent geometry