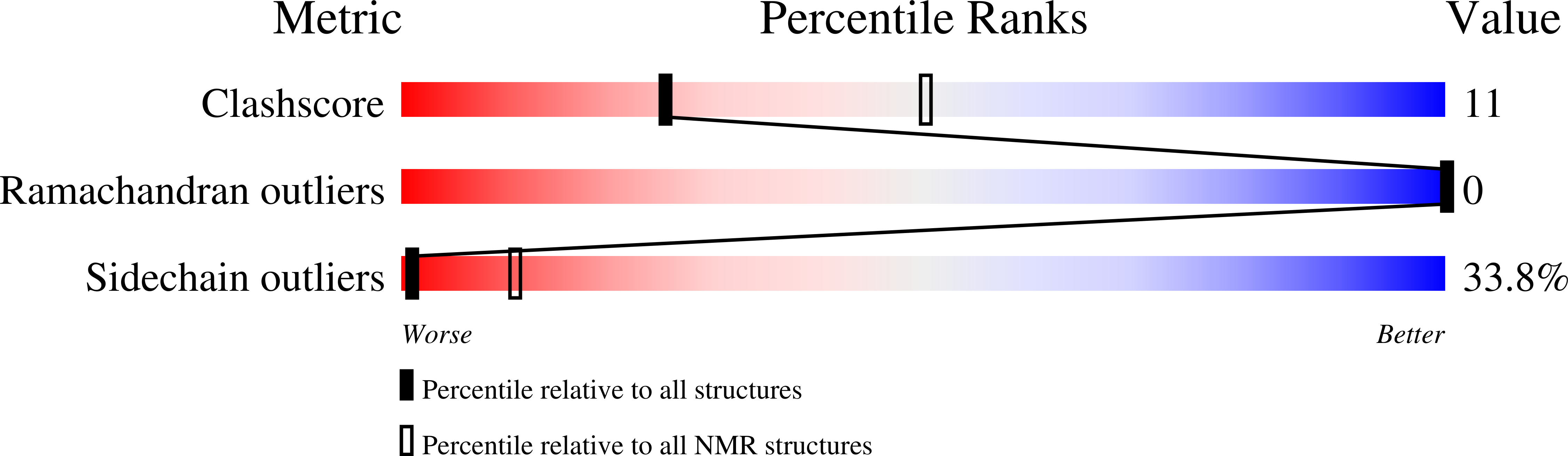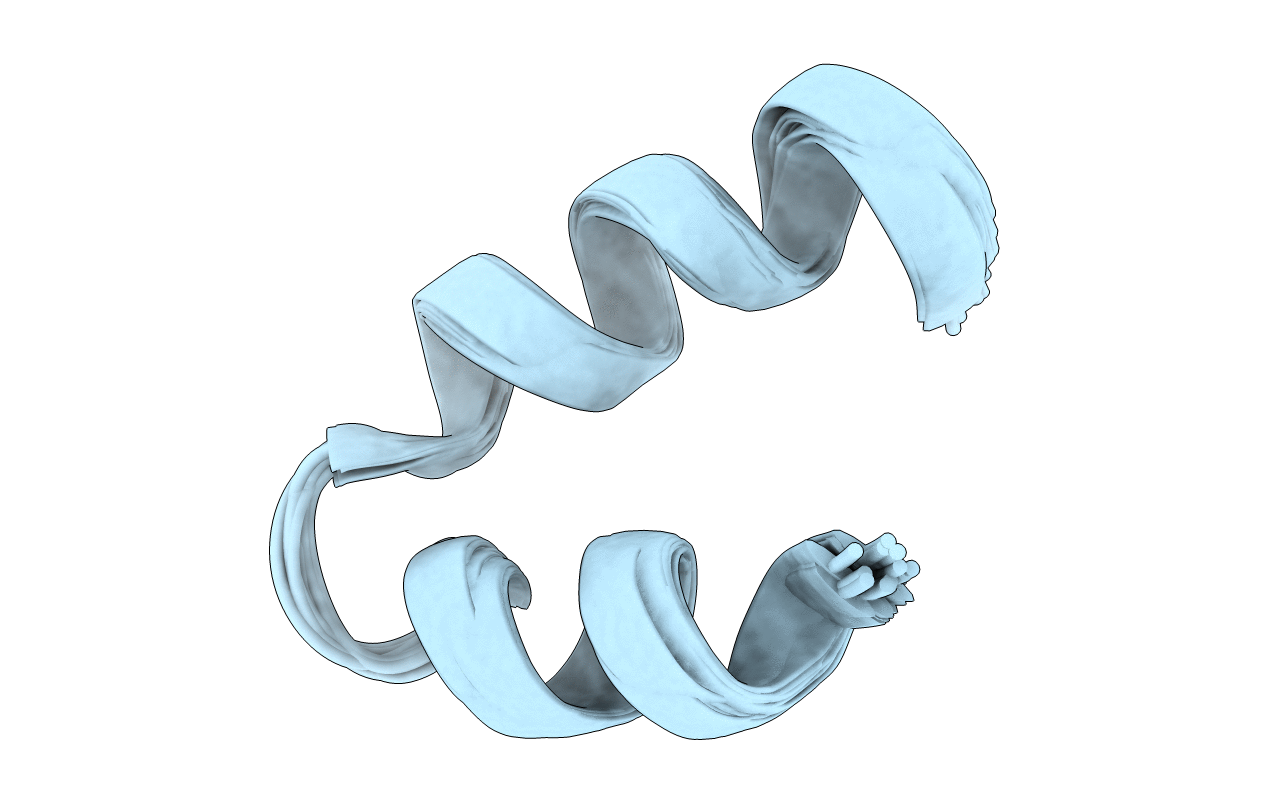
Deposition Date
2008-09-30
Release Date
2009-02-10
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2K98
Keywords:
Title:
Helical hairpin structure of potent antimicrobial peptide MSI-594 in the presence of Lipopolysaccharide micelle
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy