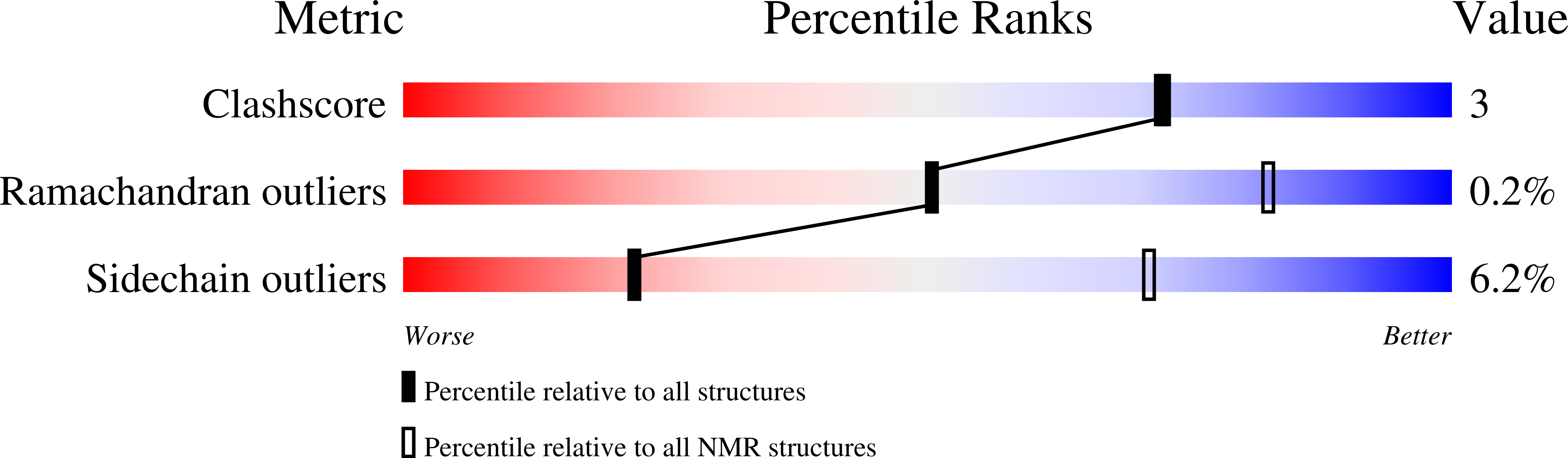
Deposition Date
2008-09-25
Release Date
2009-04-28
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2K8X
Keywords:
Title:
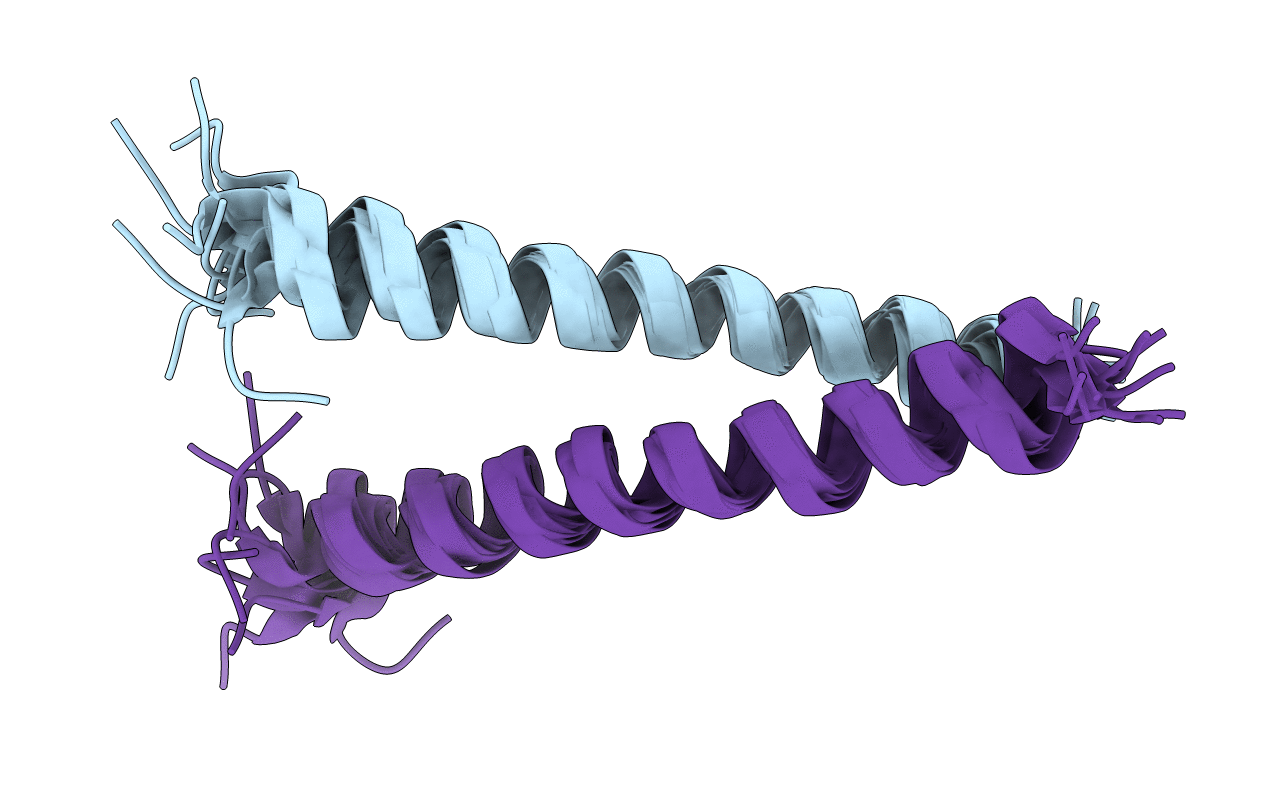
GlyTM1b(1-19)zip: A Chimeric Peptide Model of the N-Terminus of a Rat Short Alpha-Tropomyosin with the N-Terminus Encoded by Exon 1b in Complex with TM9d(252-284), a Peptide Model Containing the C Terminus of Alpha-Tropomyosin Encoded by Exon 9d
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
10
Selection Criteria:
target function