Abstact
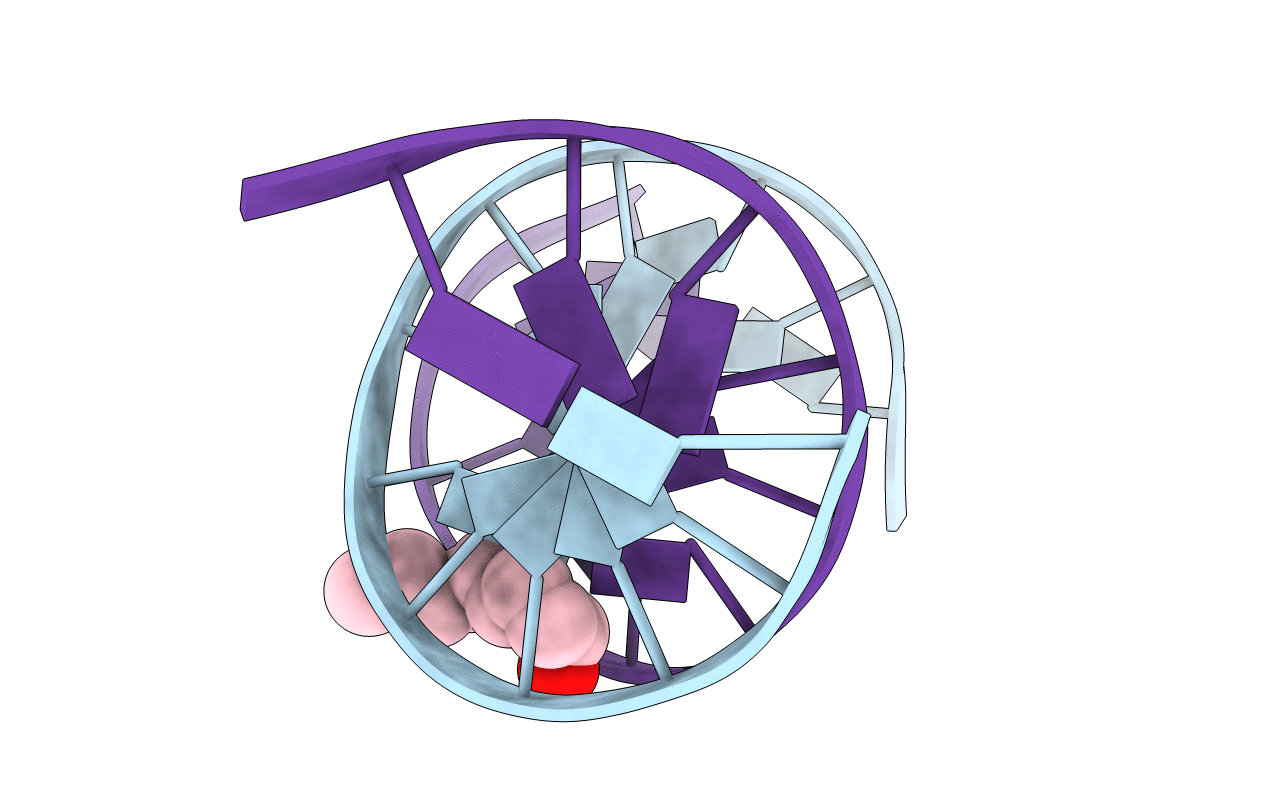
The trans-4-hydroxynonenal (HNE)-derived exocyclic 1, N(2)-dG adduct with (6S,8R,11S) stereochemistry forms interstrand N(2)-dG-N(2)-dG cross-links in the 5'-CpG-3' DNA sequence context, but the corresponding adduct possessing (6R,8S,11R) stereochemistry does not. Both exist primarily as diastereomeric cyclic hemiacetals when placed into duplex DNA [Huang, H., Wang, H., Qi, N., Kozekova, A., Rizzo, C. J., and Stone, M. P. (2008) J. Am. Chem. Soc. 130, 10898-10906]. To explore the structural basis for this difference, the HNE-derived diastereomeric (6S,8R,11S) and (6R,8S,11R) cyclic hemiacetals were examined with respect to conformation when incorporated into 5'-d(GCTAGC XAGTCC)-3' x 5'-d(GGACTCGCTAGC)-3', containing the 5'-CpX-3' sequence [X = (6S,8R,11S)- or (6R,8S,11R)-HNE-dG]. At neutral pH, both adducts exhibited minimal structural perturbations to the DNA duplex that were localized to the site of the adduction at X(7) x C(18) and its neighboring base pair, A(8) x T(17). Both the (6S,8R,11S) and (6R,8S,11R) cyclic hemiacetals were located within the minor groove of the duplex. However, the respective orientations of the two cyclic hemiacetals within the minor groove were dependent upon (6S) versus (6R) stereochemistry. The (6S,8R,11S) cyclic hemiacetal was oriented in the 5'-direction, while the (6R,8S,11R) cyclic hemiacetal was oriented in the 3'-direction. These cyclic hemiacetals effectively mask the reactive aldehydes necessary for initiation of interstrand cross-link formation. From the refined structures of the two cyclic hemiacetals, the conformations of the corresponding diastereomeric aldehydes were predicted, using molecular mechanics calculations. Potential energy minimizations of the duplexes containing the two diastereomeric aldehydes predicted that the (6S,8R,11S) aldehyde was oriented in the 5'-direction while the (6R,8S,11R) aldehyde was oriented in the 3'-direction. These stereochemical differences in orientation suggest a kinetic basis that explains, in part, why the (6S,8R,11S) stereoisomer forms interchain cross-links in the 5'-CpG-3' sequence whereas the (6R,8S,11R) stereoisomer does not.