Deposition Date
2008-08-06
Release Date
2008-08-19
Last Version Date
2024-05-22
Entry Detail
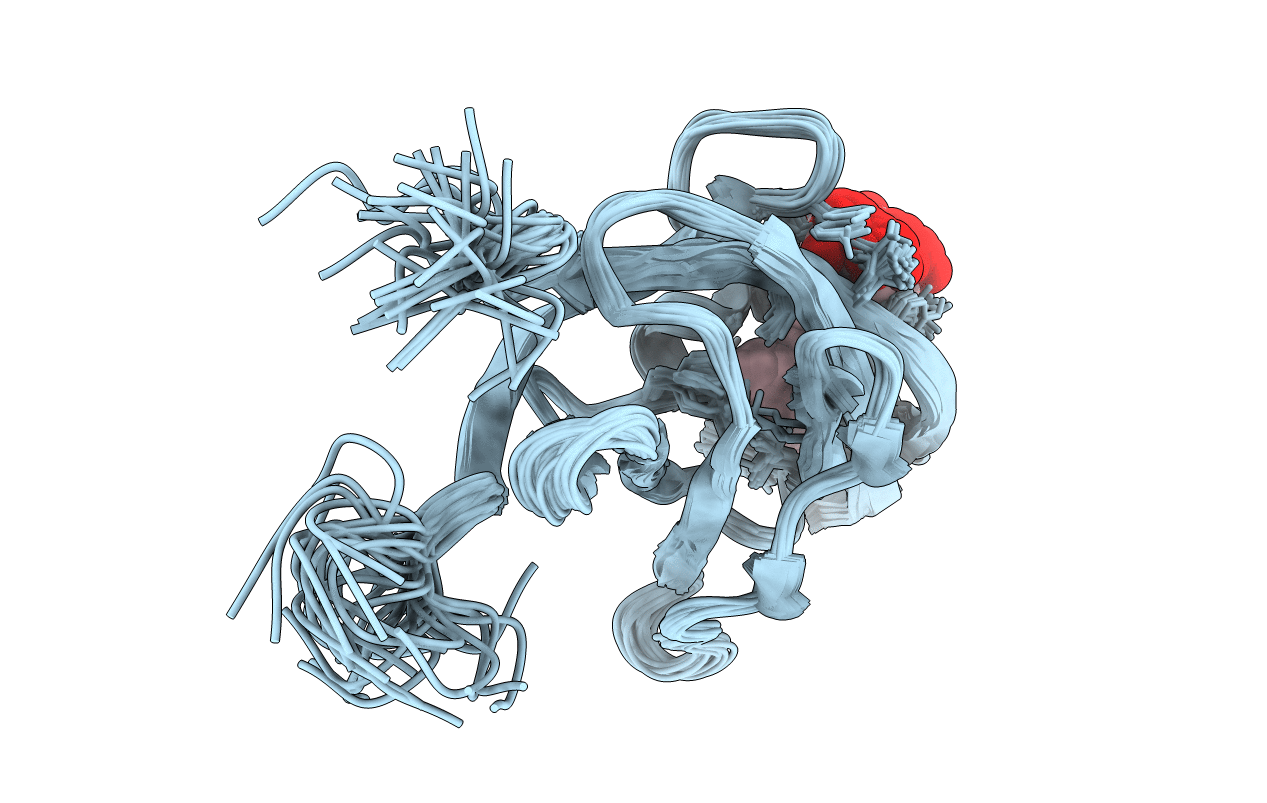
PDB ID:
2K78
Keywords:
Title:
Solution Structure of the IsdC NEAT domain bound to Zinc Protoporphyrin
Biological Source:
Source Organism(s):
Staphylococcus aureus (strain MW2) (Taxon ID: 196620)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
30
Selection Criteria:
structures with the lowest energy


