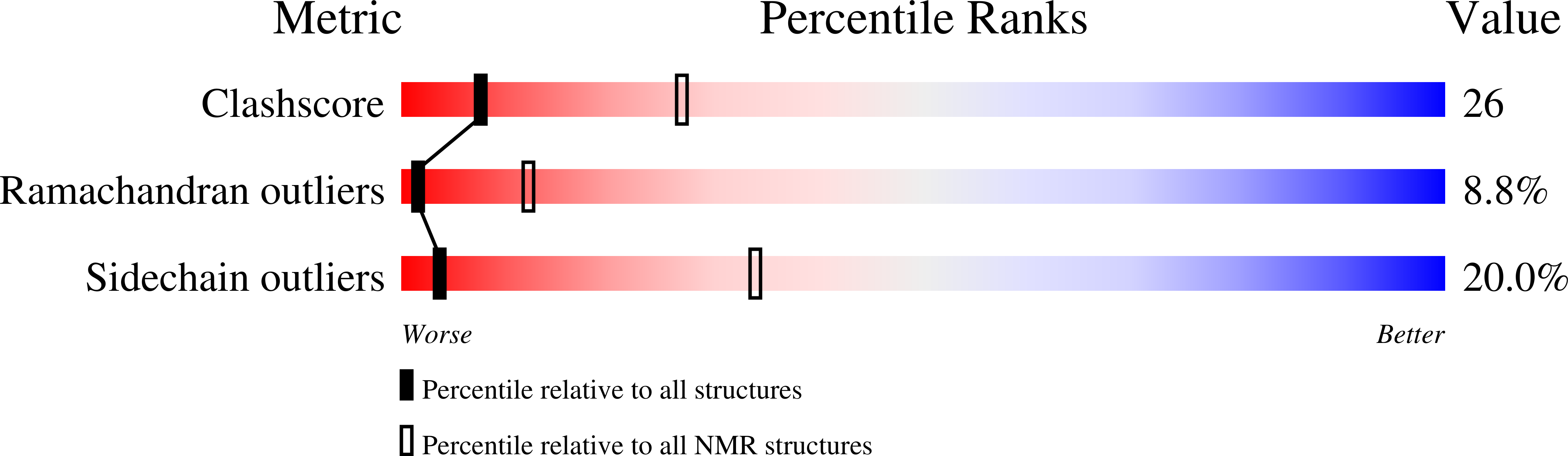
Deposition Date
2008-07-24
Release Date
2008-12-16
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2K6U
Keywords:
Title:
The Solution Structure of a Conformationally Restricted Fully Active Derivative of the Human Relaxin-like Factor (RLF)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy