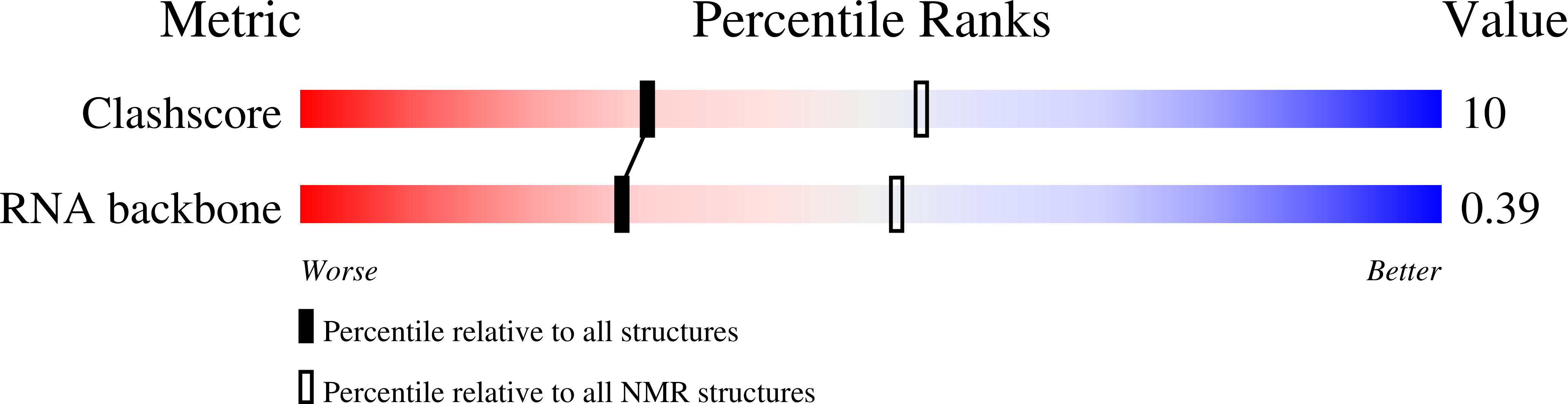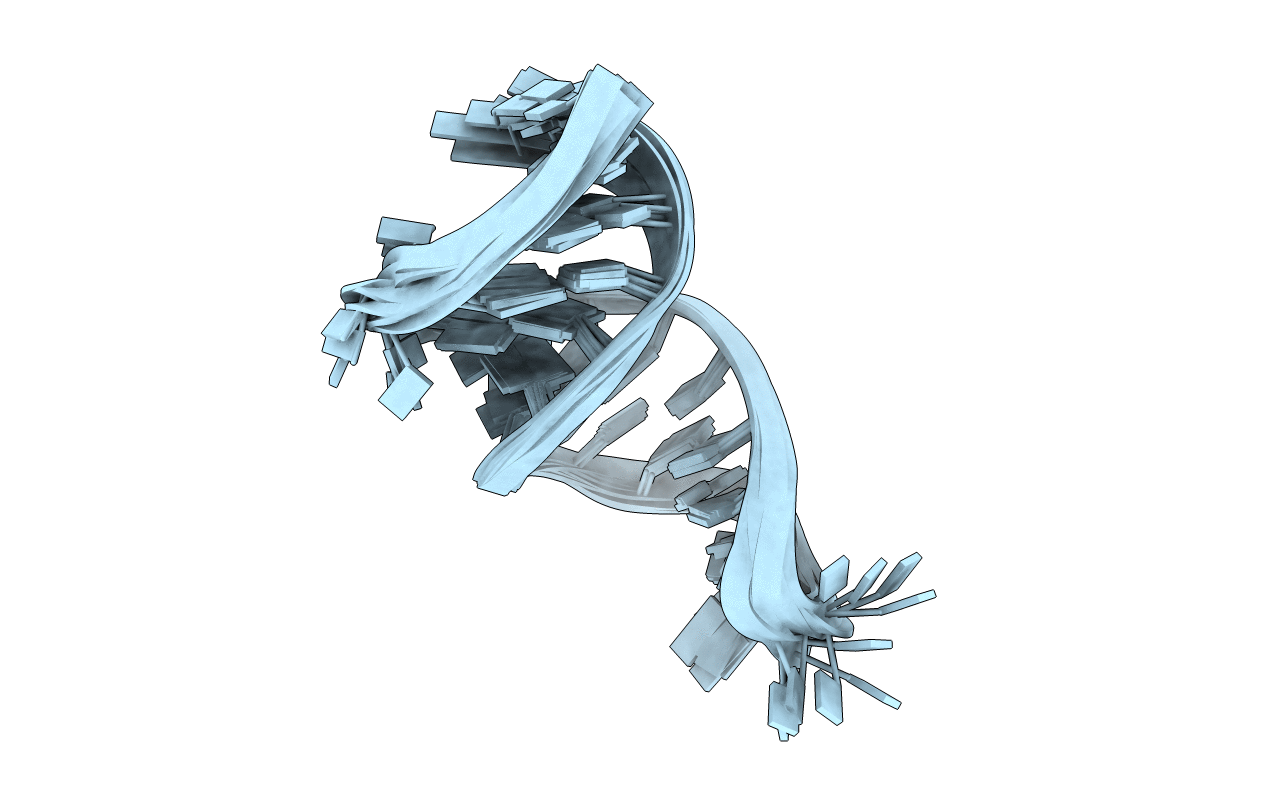
Deposition Date
2008-07-01
Release Date
2009-07-14
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2K5Z
Keywords:
Title:
Solution structure and dynamics of the apical stem-loop of Duck hepatitis B virus
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy