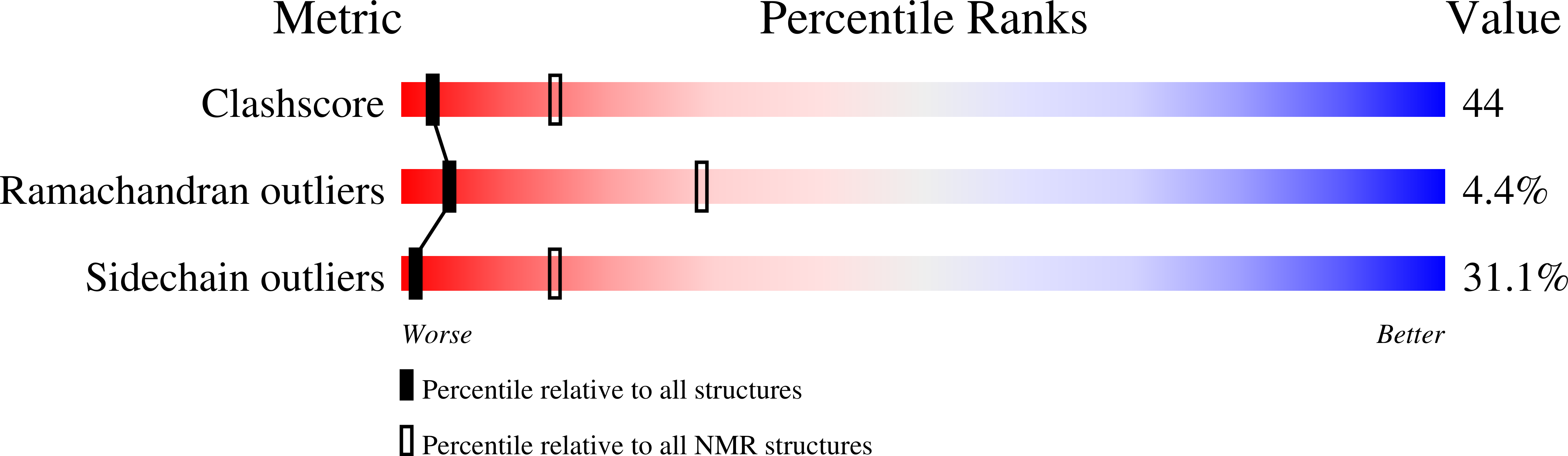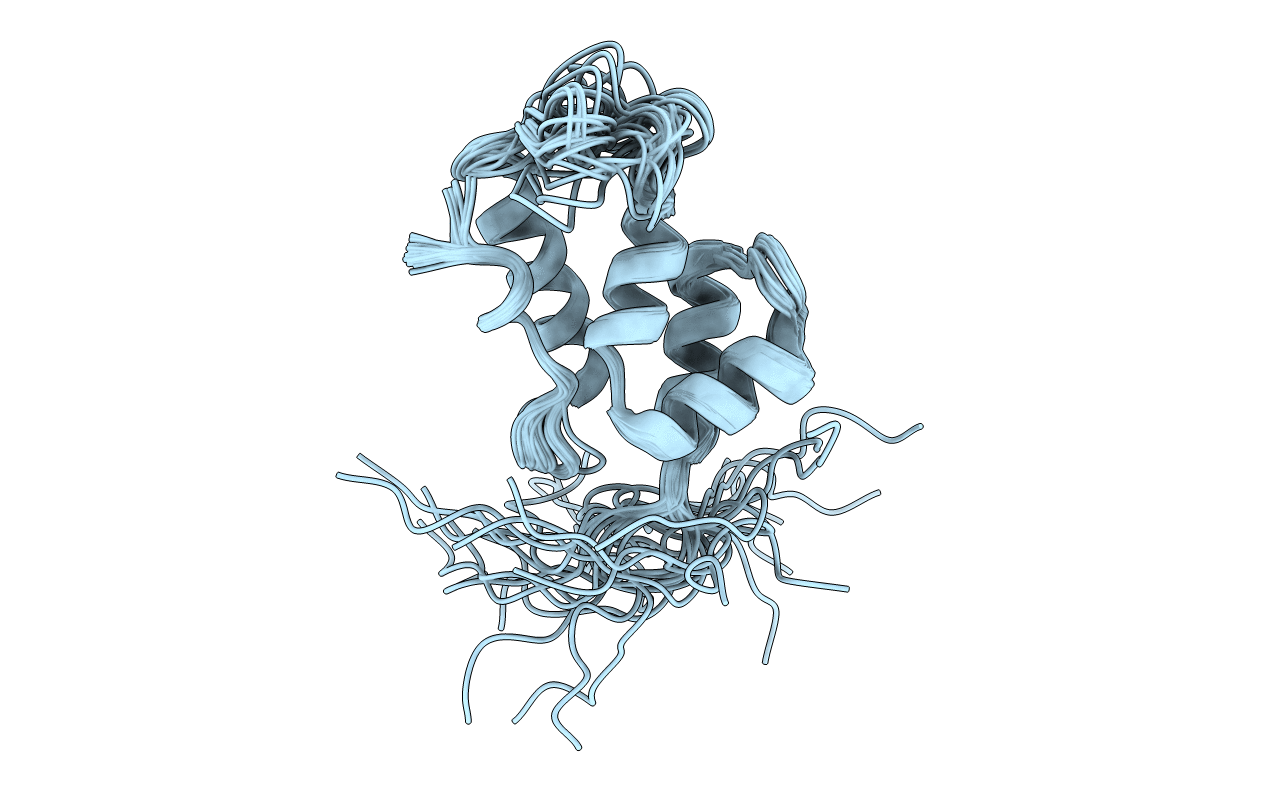
Deposition Date
2007-12-13
Release Date
2008-02-26
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2JYG
Keywords:
Title:
Solution Structure of the W184A/M185A Mutant of the Carboxy-terminal Dimerization Domain of the HIV-1 Capsid Protein
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
500
Conformers Submitted:
30
Selection Criteria:
target function