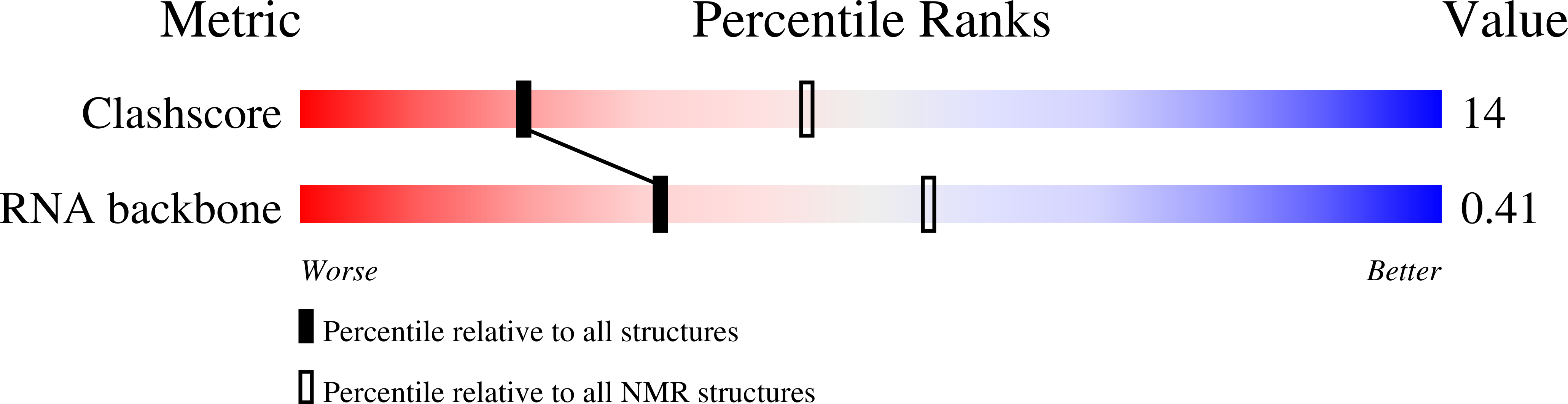
Deposition Date
2007-07-03
Release Date
2007-11-20
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2JSE
Keywords:
Title:
NMR reveals absence of hydrogen bonding in adjacent UU and AG mismatches in an isolated internal loop from ribosomal RNA.
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy and least distance and angle violations