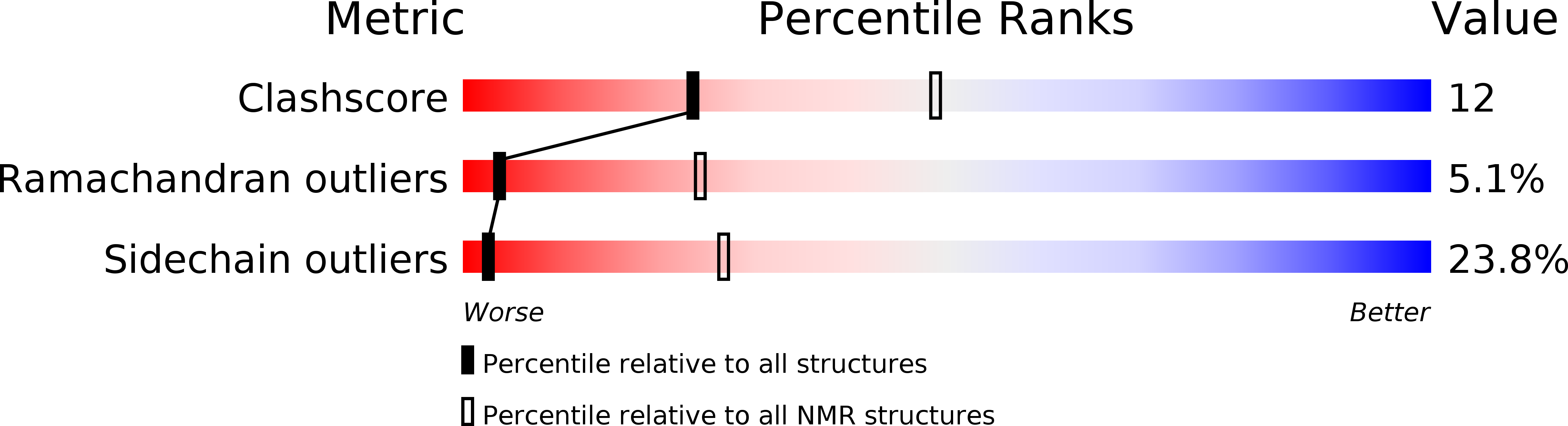Deposition Date
2007-06-20
Release Date
2008-05-27
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2JR3
Keywords:
Title:
Antibacterial Peptide from Eggshell Matrix: Structure and Self-assembly of beta-defensin Like Peptide from the Chinese Soft-shelled Turtle Eggshell
Biological Source:
Source Organism(s):
Pelodiscus sinensis (Taxon ID: )
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy