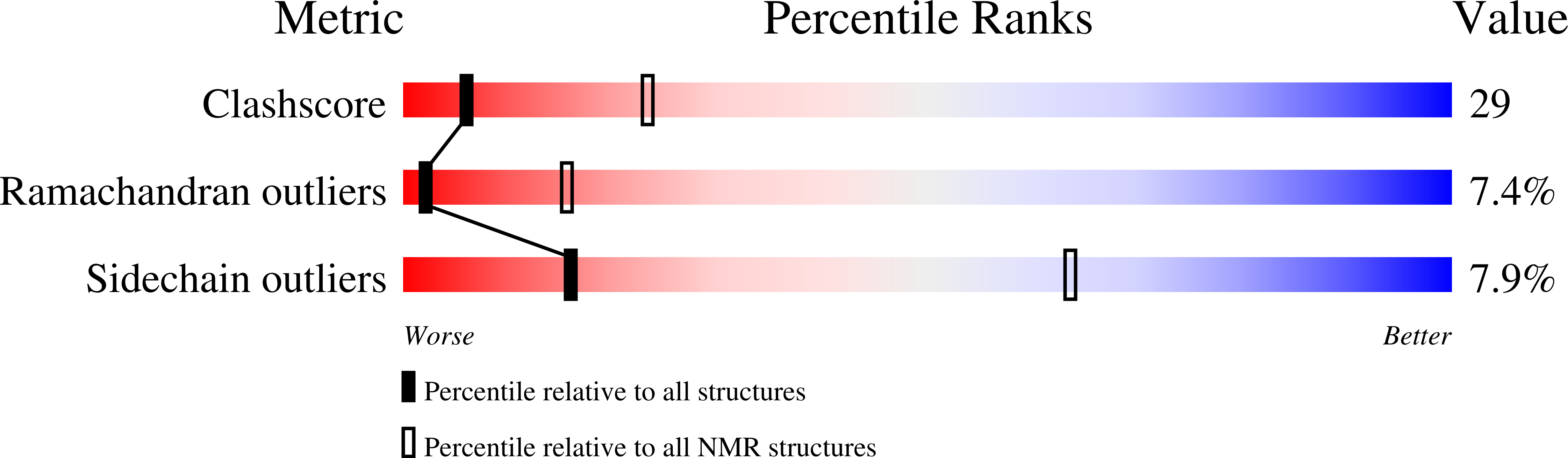
Deposition Date
2007-03-14
Release Date
2007-09-18
Last Version Date
2023-12-20
Entry Detail
PDB ID:
2JOK
Keywords:
Title:
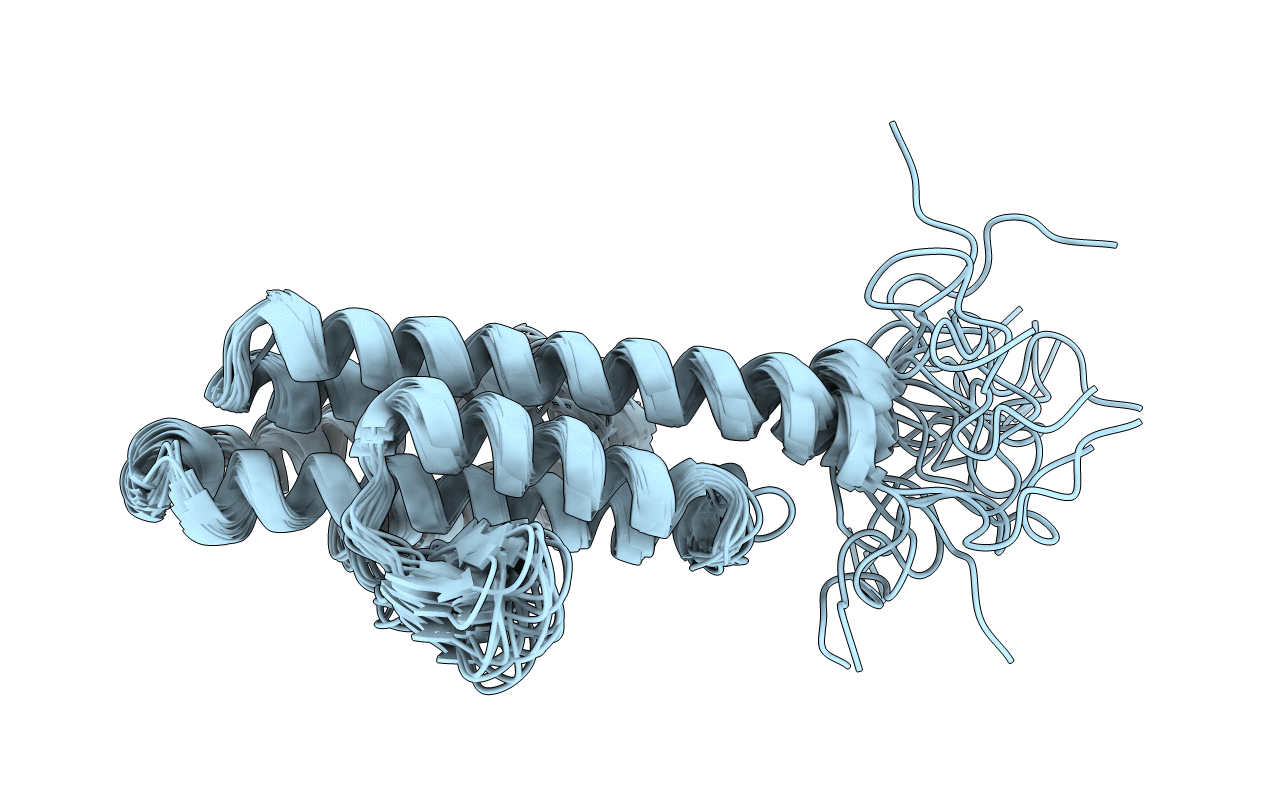
NMR structure of the catalytic domain of guanine nucleotide exchange factor BopE from Burkholderia pseudomallei
Biological Source:
Source Organism(s):
Burkholderia pseudomallei (Taxon ID: 28450)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy