Deposition Date
2007-01-16
Release Date
2007-10-30
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2JEC
Keywords:
Title:
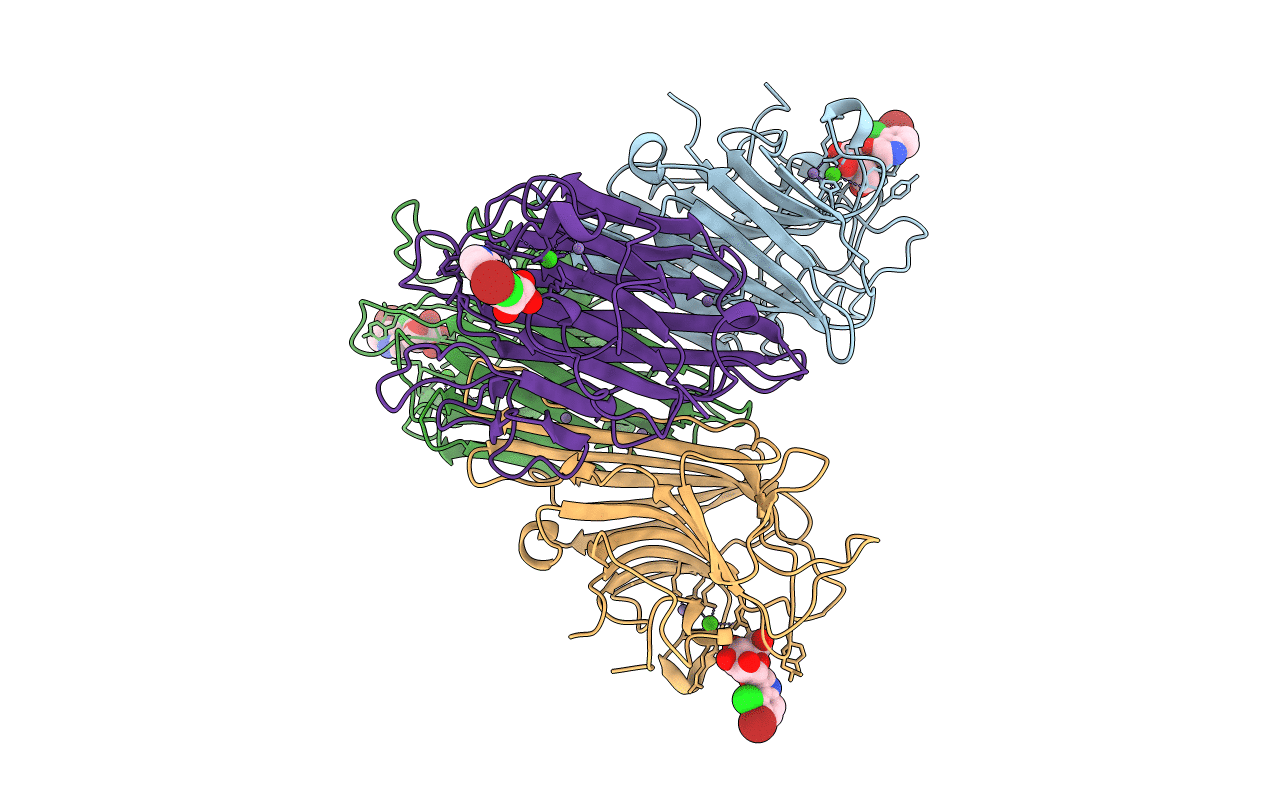
crystal structure of recombinant DiocleA grandiflora lectin mutant E123A-H131N-K132Q complexed witH 5-bromo-4-chloro-3-indolyl-a-D- mannose
Biological Source:
Source Organism(s):
DIOCLEA GRANDIFLORA (Taxon ID: 3837)
Expression System(s):
Method Details:
Experimental Method:
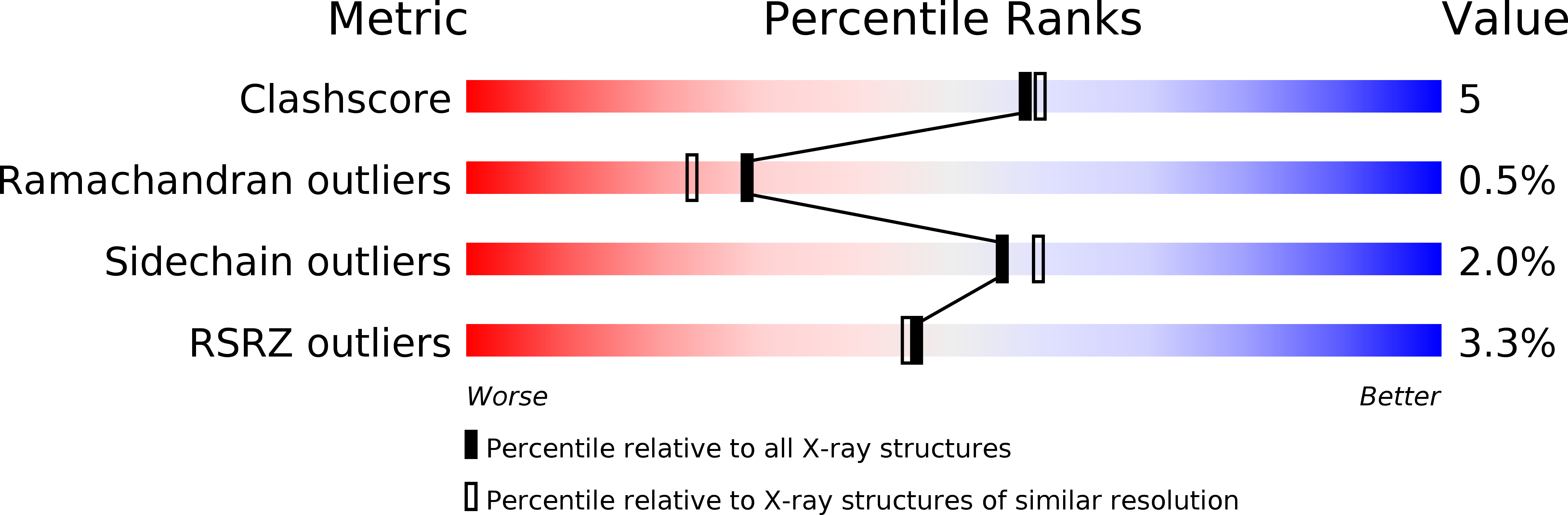
Resolution:
2.00 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 21 21 21