Deposition Date
2006-11-23
Release Date
2007-02-20
Last Version Date
2025-12-17
Entry Detail
PDB ID:
2JA5
Keywords:
Title:
CPD lesion containing RNA Polymerase II elongation complex A
Biological Source:
Source Organism(s):
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Saccharomyces cerevisiae (Taxon ID: 4932)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Saccharomyces cerevisiae (Taxon ID: 4932)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Method Details:
Experimental Method:
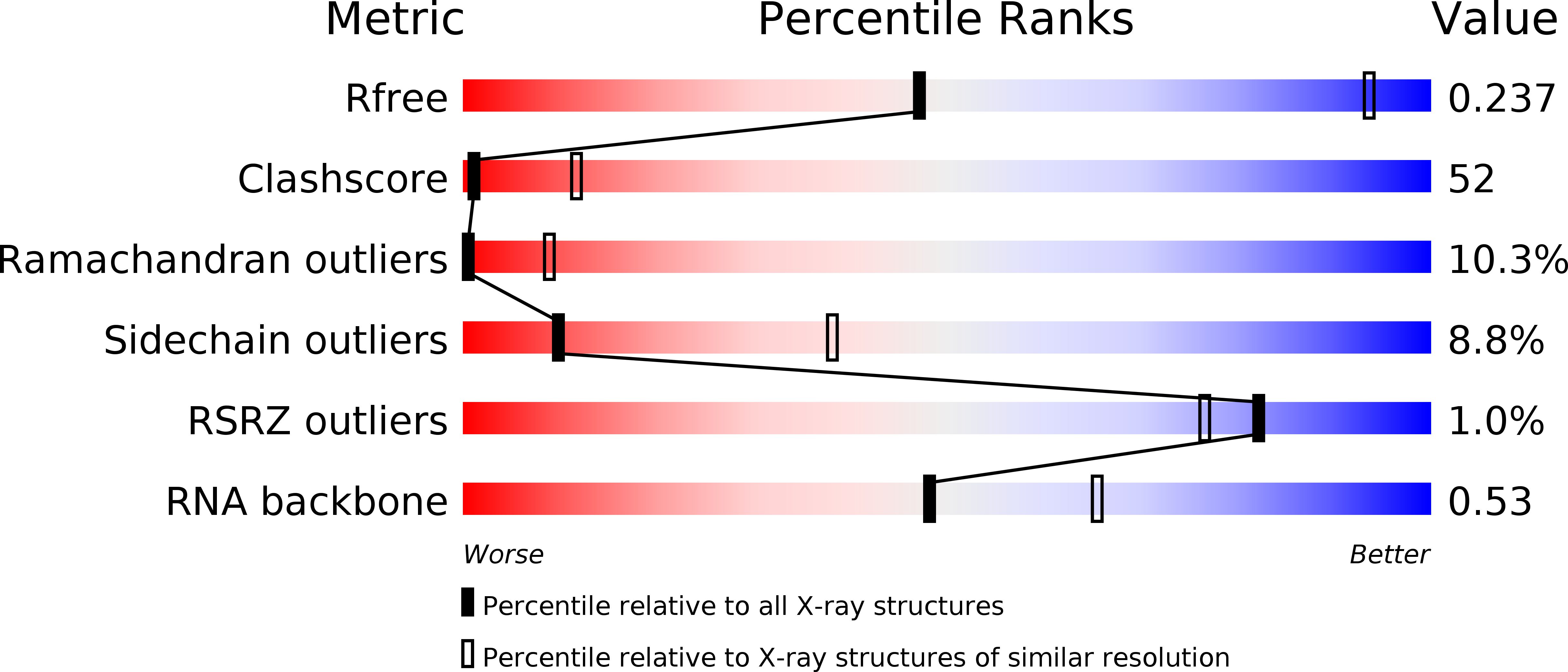
Resolution:
3.80 Å
R-Value Free:
0.27
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
C 2 2 21