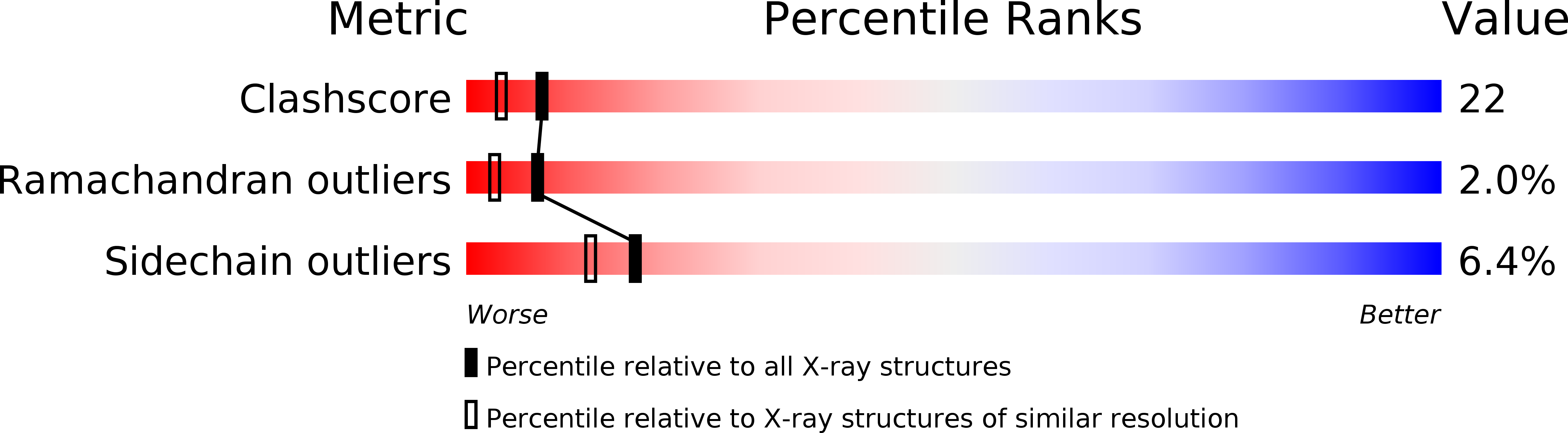
Deposition Date
1990-01-02
Release Date
1990-04-15
Last Version Date
2024-02-21
Entry Detail
PDB ID:
2I1B
Keywords:
Title:
CRYSTALLOGRAPHIC REFINEMENT OF INTERLEUKIN-1 BETA AT 2.0 ANGSTROMS RESOLUTION
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details: