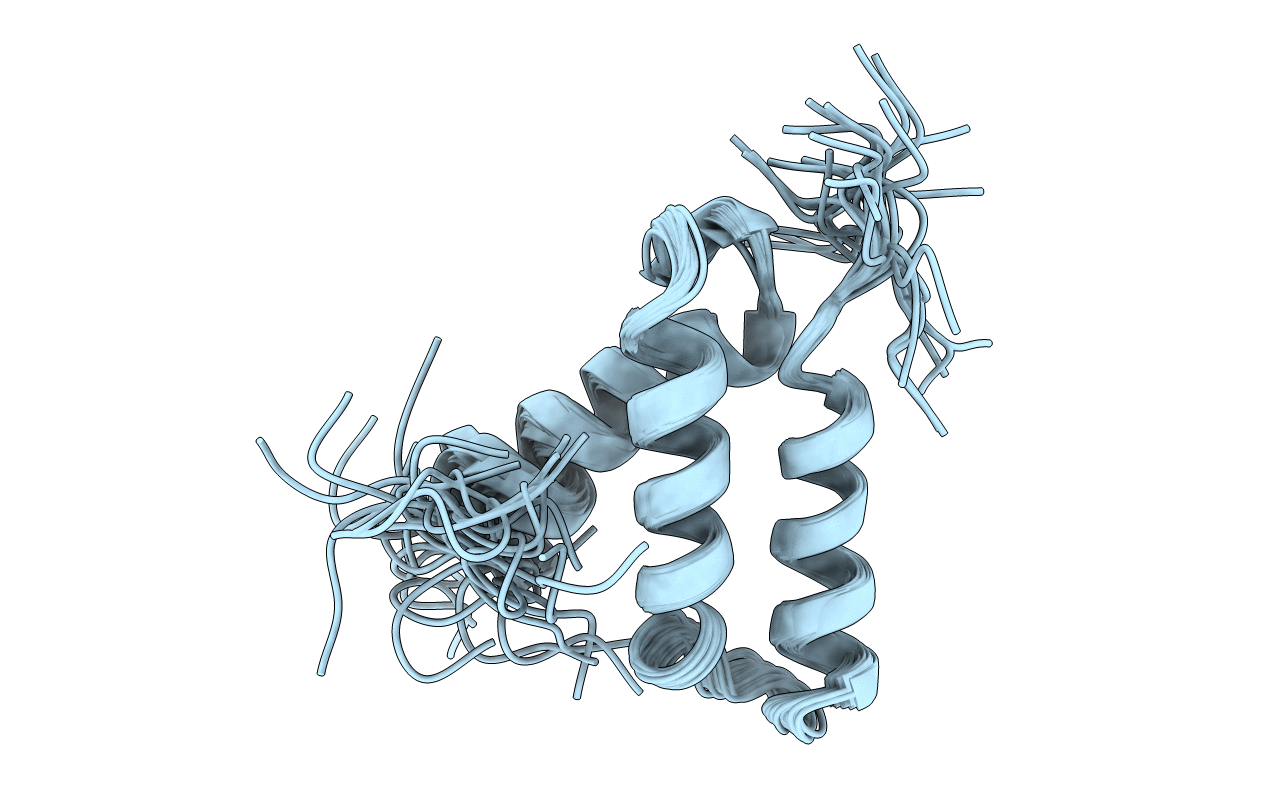
Deposition Date
1997-08-26
Release Date
1998-03-04
Last Version Date
2024-10-23
Entry Detail
PDB ID:
2HP8
Keywords:
Title:
SOLUTION STRUCTURE OF HUMAN P8-MTCP1, A CYSTEINE-RICH PROTEIN ENCODED BY THE MTCP1 ONCOGENE,REVEALS A NEW ALPHA-HELICAL ASSEMBLY MOTIF, NMR, 30 STRUCTURES
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
30
Selection Criteria:
structures with the least restraint violations


