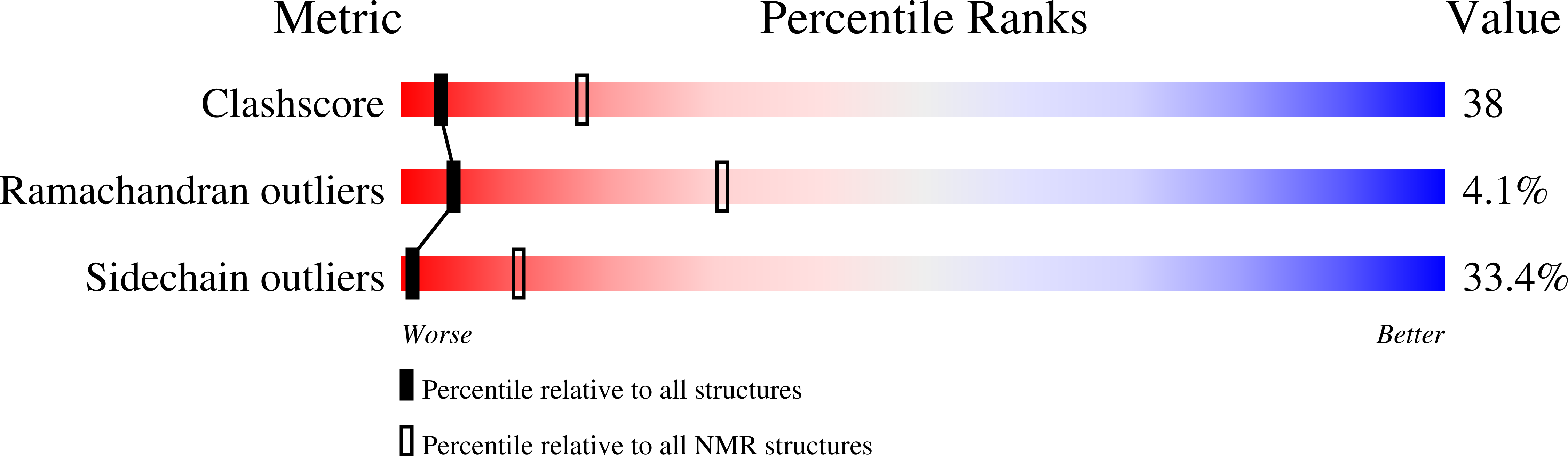
Deposition Date
1998-01-27
Release Date
1998-06-17
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2HFH
Keywords:
Title:
THE NMR STRUCTURES OF A WINGED HELIX PROTEIN: GENESIS, 20 STRUCTURES
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
LOWEST TARGET FUNCTION