Deposition Date
2006-05-03
Release Date
2007-05-08
Last Version Date
2024-05-29
Entry Detail
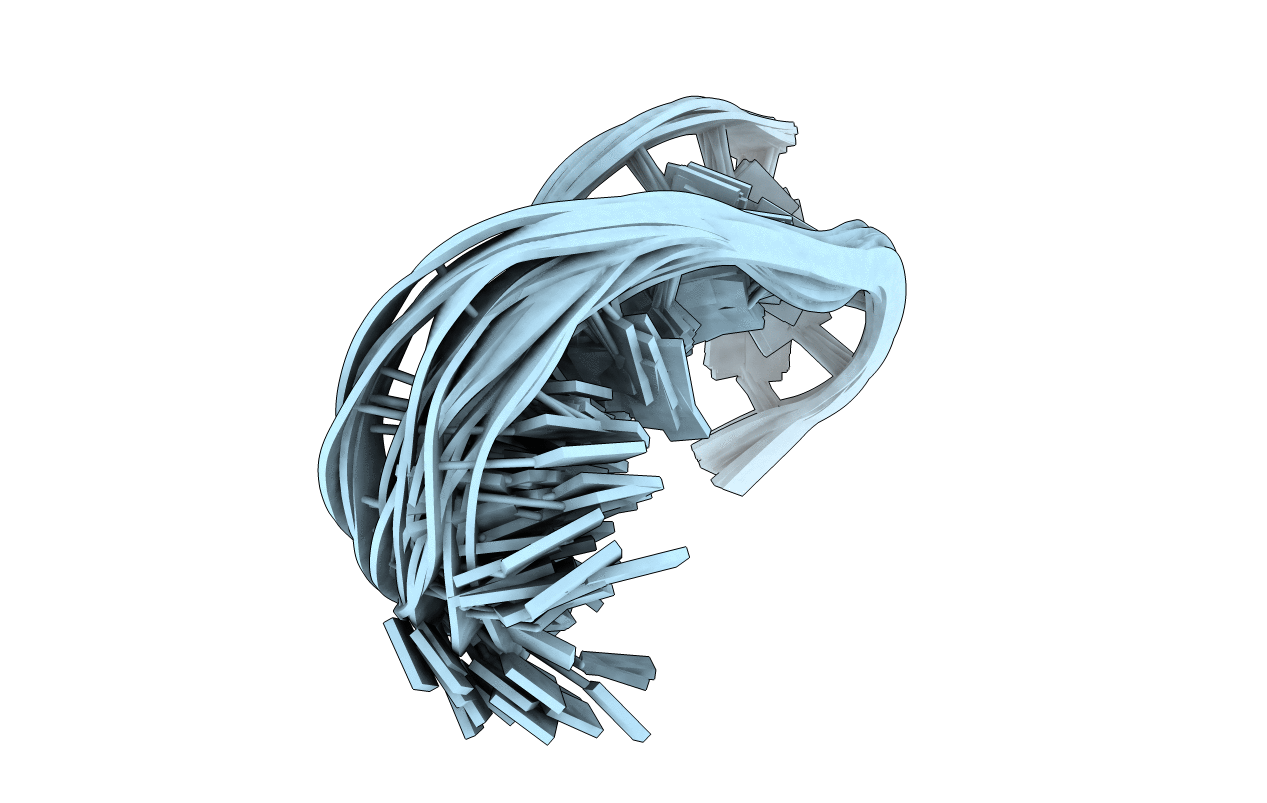
PDB ID:
2GVO
Keywords:
Title:
Solution structure of a purine rich hexaloop hairpin belonging to PGY/MDR1 mRNA and targeted by antisense oligonucleotides
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
21
Selection Criteria:
all calculated structures submitted, back calculated data agree with experimental NOESY spectrum, structures with acceptable covalent geometry, structures with favorable non-bond energy,structures with the least restraint violations, structures with the lowest energy


