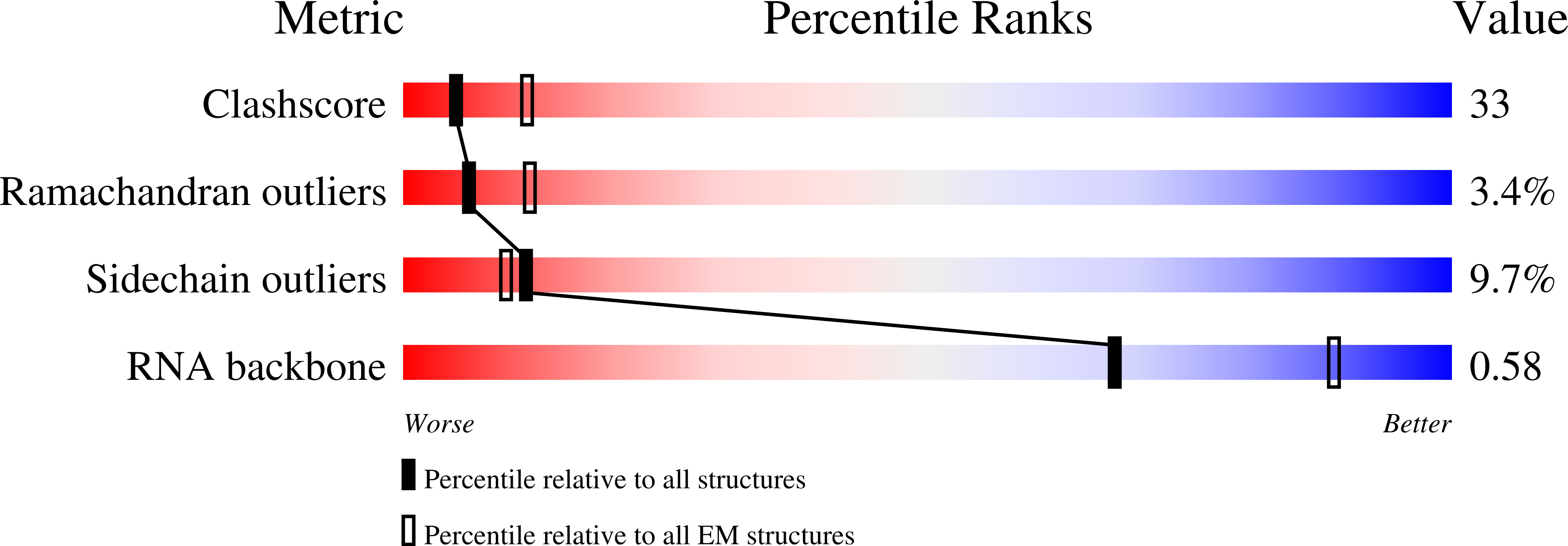
Deposition Date
2006-04-12
Release Date
2006-06-13
Last Version Date
2024-10-09
Entry Detail
PDB ID:
2GO5
Keywords:
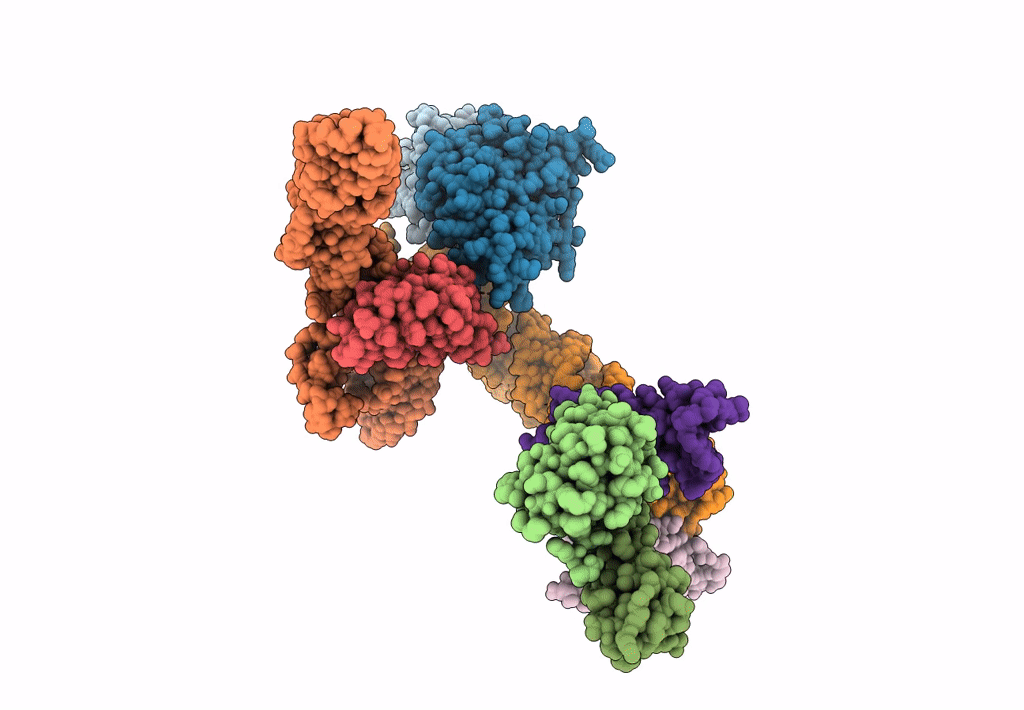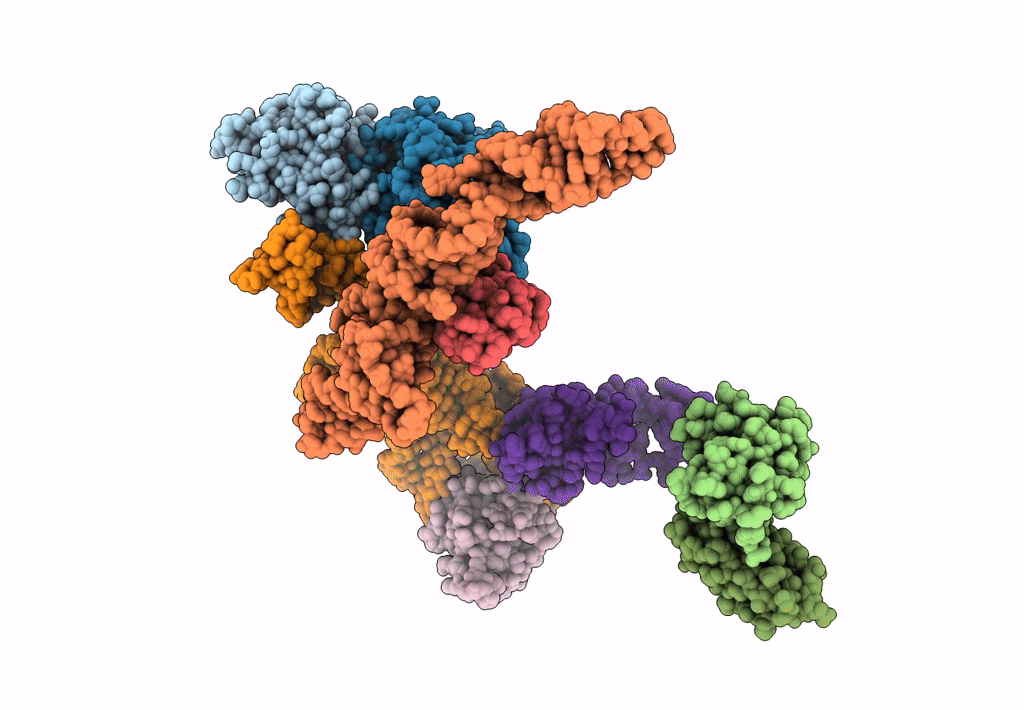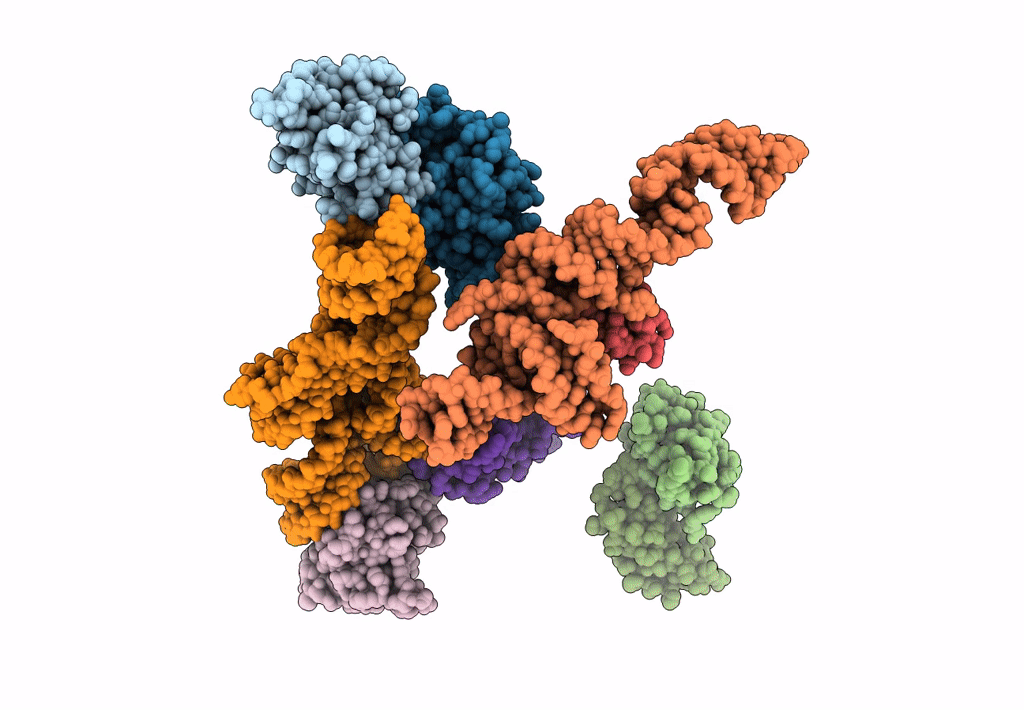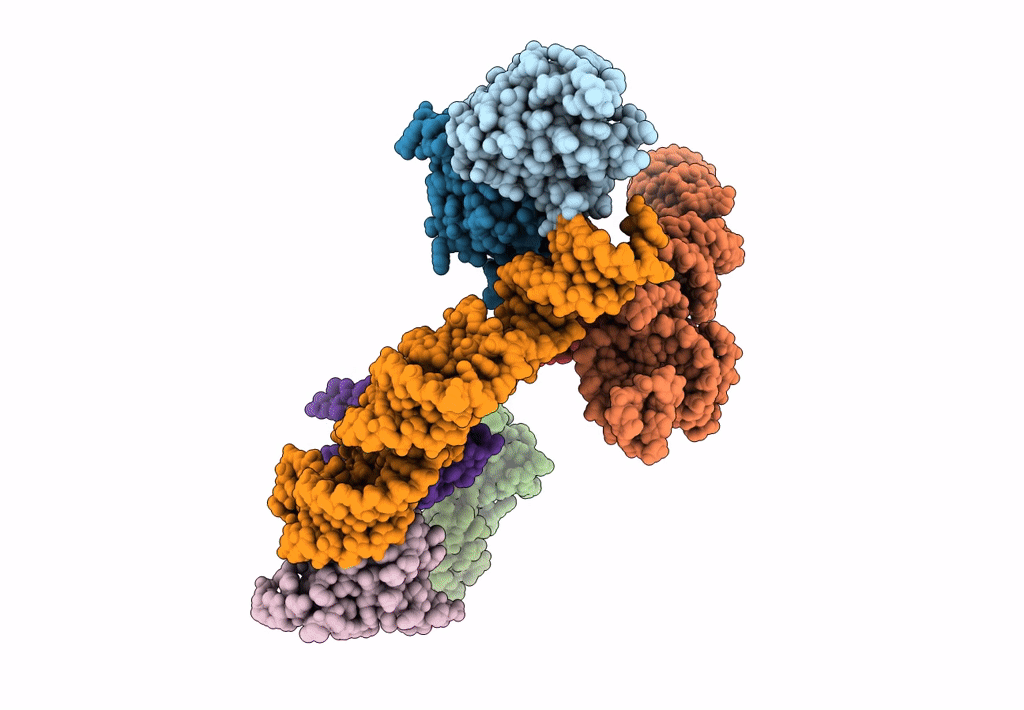
Title:
Structure of signal recognition particle receptor (SR) in complex with signal recognition particle (SRP) and ribosome nascent chain complex
Biological Source:
Source Organism(s):
Canis sp. (Taxon ID: 9616)
Homo sapiens (Taxon ID: 9606)
Mus musculus (Taxon ID: 10090)
Triticum sp. (Taxon ID: 4569)
Homo sapiens (Taxon ID: 9606)
Mus musculus (Taxon ID: 10090)
Triticum sp. (Taxon ID: 4569)
Method Details:
Experimental Method:
Resolution:
7.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE