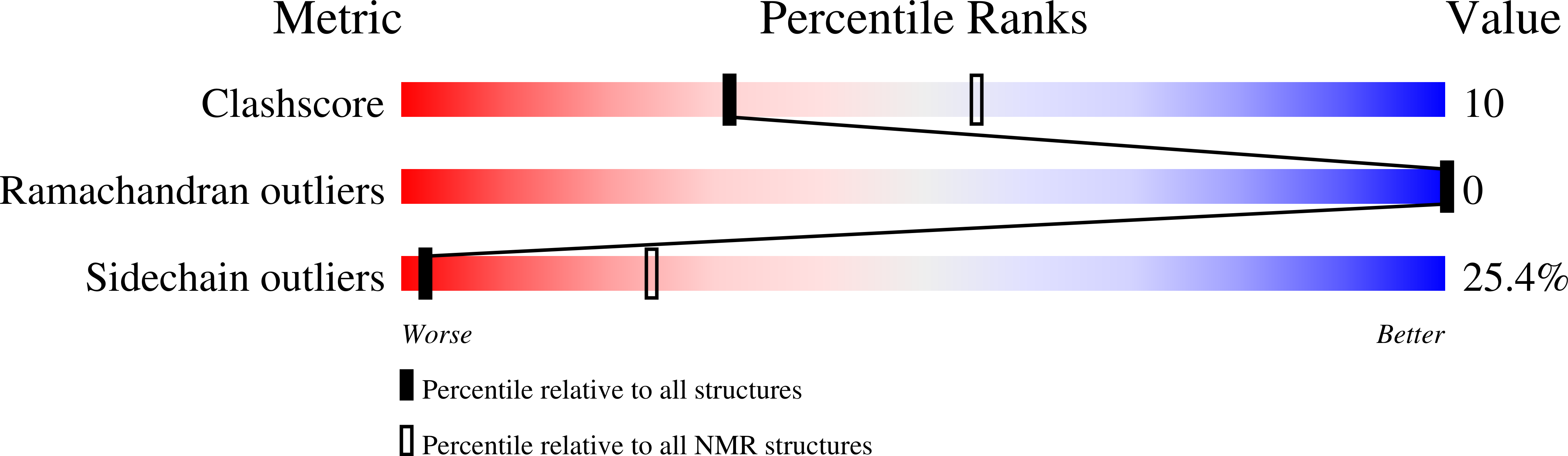
Deposition Date
2006-03-15
Release Date
2006-09-19
Last Version Date
2024-05-29
Entry Detail
Method Details:
Experimental Method:
Conformers Calculated:
300
Conformers Submitted:
14
Selection Criteria:
14 convergent conformers are presented having the lowest energy and the best structural quality in Ramachadran plot