Deposition Date
2006-03-03
Release Date
2006-04-25
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2G8U
Keywords:
Title:
B. halodurans RNase H catalytic domain D132N mutant in complex with Mg2+ and RNA/DNA hybrid (non-P nick at the active site)
Biological Source:
Source Organism(s):
Bacillus halodurans (Taxon ID: 86665)
Expression System(s):
Method Details:
Experimental Method:
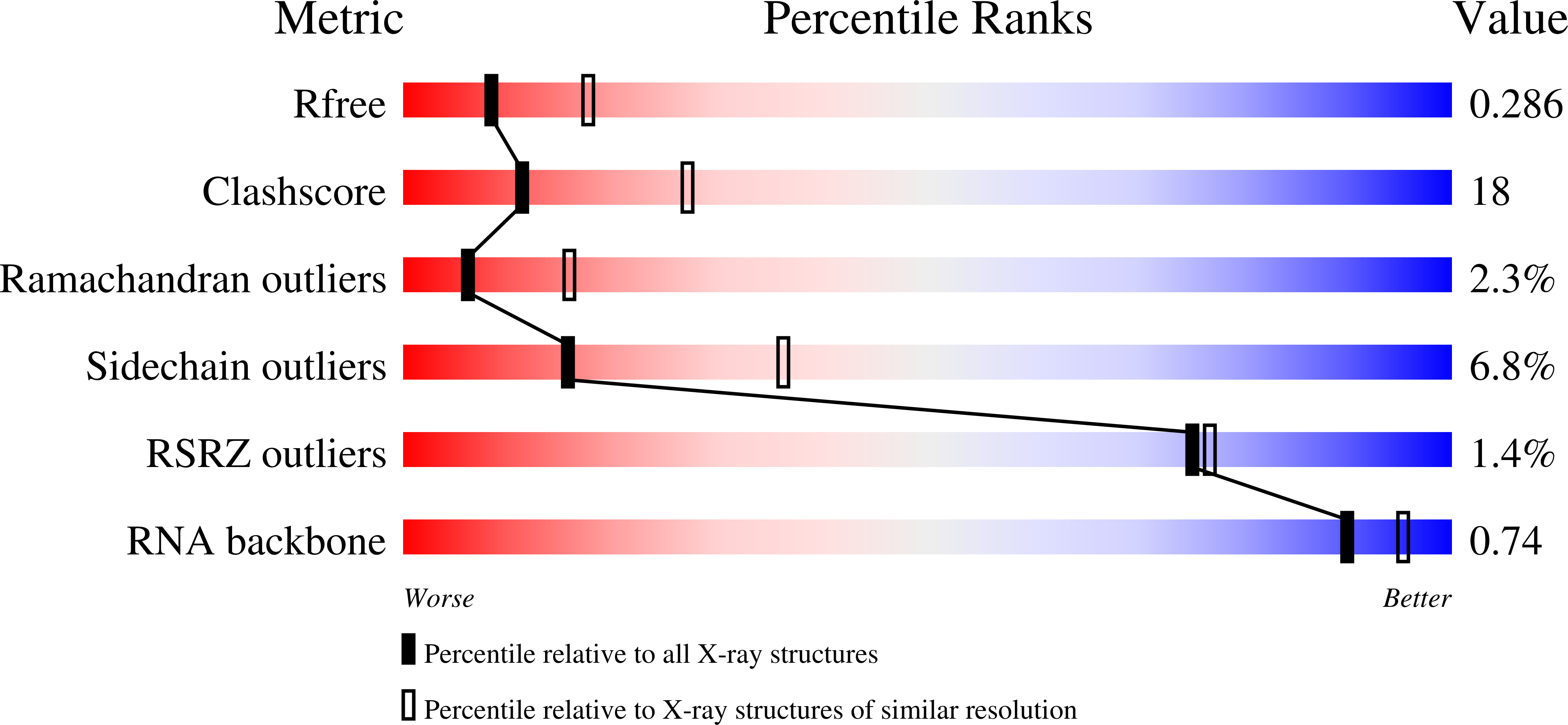
Resolution:
2.70 Å
R-Value Free:
0.28
R-Value Work:
0.22
Space Group:
C 1 2 1